| "Descrizione" by Ark90 (12432 pt) | 2024-Jun-10 14:34 |
Review Consensus: 16 Rating: 8 Number of users: 2
| Evaluation | N. Experts | Evaluation | N. Experts |
|---|---|---|---|
| 1 | 6 | ||
| 2 | 7 | ||
| 3 | 8 | ||
| 4 | 9 | ||
| 5 | 10 |
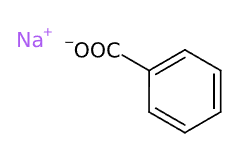
Sodium Benzoate is a chemical compound, organic sodium salt of benzoic acid.
The name defines the structure of the molecule
- "Sodium" is an alkali metal, symbol Na, with atomic number 11. It's an essential element for life and plays a key role in regulating blood pressure and plasma volume.
- "Benzoate" indicates the presence of a benzoate group, which is an ester of benzoic acid.
Description and function of the raw materials used in production
- Benzoic acid - An aromatic carboxylic acid found in many plants and resins, but for industrial use, it is often produced synthetically.
- Sodium hydroxide (or sodium carbonate) - Used to neutralize benzoic acid and form the sodium salt.
Summary of the industrial synthesis process step by step
- Neutralization - Benzoic acid is neutralized with sodium hydroxide or sodium carbonate to form sodium benzoate and water.
- Crystallization - The resulting solution is cooled to form crystals of sodium benzoate.
- Purification - The crystals are filtered, washed, and dried to obtain pure sodium benzoate.
It appears as a white, fine, odourless powder. Soluble in water, soluble in ethanol.

What it is used for and where it is used
Food
It is used as a preservative in the food industry and is labelled with the number E211 in the list of European food additives. The antibacterial effectiveness of benzoic acid and its salts, such as sodium benzoate in this case, is highly dependent on the pH of the substances in which it is added. Sodium benzoate is used intensively in soft drinks containing high-fructose corn syrup because of its power to increase acidity and taste. Because of these characteristics it is frequently found in sauces, jams, fruit juices and pickles. It is also usually found in foods containing vinegar. In minimal quantities it is found in vegetables, fruit, meat, dairy products.
Binding agent in confectionery products, as a buffer improves quality in bread.
In beer it improves diastatic quality and fermentation capacity.
Cosmetics
It is a restricted ingredient as V/1 a Relevant Item in the Annexes of the European Cosmetics Regulation 1223/2009. Substance or ingredient reported: Benzoic acid and its sodium salt
Used in body care products, facial care products for its anti-bacterial and anti-fungal properties.
Fragrance. It plays a very important role in the formulation of cosmetic products as it allows perfume to be enhanced, masked or added to the final product, improving its commercial viability. The consumer always expects to find a pleasant scent in a cosmetic product.
Preservative. Any product containing organic, inorganic compounds, water, needs to be preserved from microbial contamination. Preservatives act against the development of harmful microorganisms and against oxidation of the product.
Medicine
In medicine sodium benzoate is credited in this study with a potential benefit for cognitive function (1) and this other study confirmed that adjuvant therapy with sodium benzoate improved the symptomatology of patients with clozapine-resistant schizophrenia (2).
Cirrhosis and congenital errors of metabolism can lead to a serious but usually reversible neuropsychiatric complication, hepatic encephalopathy. Sodium benzoate is an inexpensive adjunct agent that can be used in addition to lactulose and rifaximin and may provide an option for selected patients with refractory hepatic encephalopathy who have not responded to standard therapies or cannot afford them (3).
High amounts of sodium benzoate may cause glycine deficiency, which may adversely affect brain neurochemistry (4).
Commercial applications
Food industry: Used as a preservative in a variety of food products, including soft drinks, fruit juices, condiments, and sauces.
Cosmetic and Skincare Products: Used as a preservative in items such as creams, lotions, and shampoos.
Pharmaceutical: Might be used as a preservative in oral and topical medications.
Other Uses: Found in oral care products like mouthwashes and toothpastes.
Properties
Preservative: Inhibits the growth of bacteria, yeast, and mold.
Water-soluble: Dissolves readily in water, making it suitable for a broad range of products.
Low toxicity profile: Generally recognized as safe (GRAS) for use in food and cosmetics at recommended concentrations.
Safety
The Cosmetic Ingredient Review Panel Expert (USA), in its final report on the safety assessment of benzyl alcohol, benzoic acid, and sodium benzoate finds that Available safety tests are not considered sufficient to support the safety of these ingredients in formulations where inhalation is a route of exposure (5).
The most relevant studies on the subject have been selected with a summary of their contents:
Typical optimal commercial product characteristics Sodium benzoate
| Appearance | White crystalline powder |
| Boiling Point | 249.3ºC at 760 mmHg |
| Melting Point | >300 °C |
| Density | 1,44 g/cm3 |
| Flash Point | 111.4ºC |
| pH (aqueous solution) | 8 |
| Acidity and alkalinity | 0.20ml/0.1N |
| Loss on drying % | ≤15 |
| Heavy metals ppm | ≤10 ≤0.001% |
| Ammonium salts ppm | ≤25 |
| Arsenic ppm | ≤3 |
| P-toluene sulfonamide | ≤10ppm |
| O-toluene sulfonamide | ≤10ppm |
| Selenium ppm | ≤30 |
| Sulphate | ≤0.1% |
| PSA | 40.13000 |
| LogP | 0.05010 |
| Water solubility | 53.0g/100ml,25℃ |
| Ethanol solubility | 1.4g/100ml |
| 1,4-Dioxane(μg/g) | 380 max |
| Benzene(μg/g) | 2 max |
| Methylene(μg/g) | 600 max |
| Safety |  |
 | |
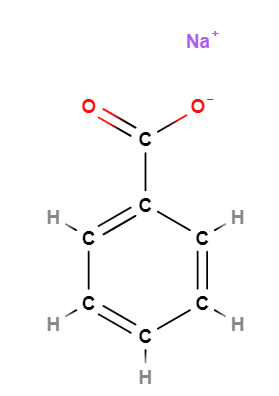 | 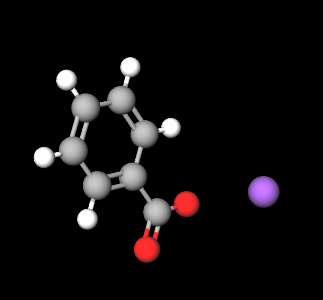 |
- Molecular Formula: C7H5O2Na o C7H5NaO2 o C6H5COONa o NaC6H5COO
- Molecular Weight: 144.105 g/mol
- Exact Mass 144.018723
- CAS: 532-32-1
- UNIII OJ245FE5EU
- EC Number 208-534-8
- DSSTox Substance ID DTXSID1020140
- IUPAC sodium;benzoate
- InChI=1S/C7H6O2.Na/c8-7(9)6-4-2-1-3-5-6;/h1-5H,(H,8,9);/q;+1/p-1
- InChl Key WXMKPNITSTVMEF-UHFFFAOYSA-M
- SMILES C1=CC=C(C=C1)C(=O)[O-].[Na+]
- MDL number MFCD00012463
- PubChem Substance ID 329823236
- ChEBI 113455
- ICSC 536
- RTECS DH6650000
- FEMA Number: 3025
- Beilstein 3572467
- NACRES NA.24
Synonyms:
- Benzoic acid, sodium salt
- E211
- Sobenate
- sodium;benzoate
- Antimol
- Carboxybenzene sodium salt
- Benzoic acid sodium salt
- Sodiumbenzoate
- Benzoate sodium
- BENZOTRON(R)
- Benzoate of soda
- PUROX S
- Benzoate, sodium
References___________________________________________________________________
(1) Lin CH, Yang HT, Chen PK, Wang SH, Lane HY. Precision Medicine of Sodium Benzoate for the Treatment of Behavioral and Psychological Symptoms of Dementia (BPSD). Neuropsychiatr Dis Treat. 2020 Feb 20;16:509-518. doi: 10.2147/NDT.S234371.
Lin CH, Chen PK, Wang SH, Lane HY. Sodium benzoate for the treatment of behavioral and psychological symptoms of dementia (BPSD): A randomized, double-blind, placebo-controlled, 6-week trial. J Psychopharmacol. 2019 Aug;33(8):1030-1033. doi: 10.1177/0269881119849815.
(2) Lin CH, Lin CH, Chang YC, Huang YJ, Chen PW, Yang HT, Lane HY. Sodium Benzoate, a D-Amino Acid Oxidase Inhibitor, Added to Clozapine for the Treatment of Schizophrenia: A Randomized, Double-Blind, Placebo-Controlled Trial. Biol Psychiatry. 2018 Sep 15;84(6):422-432. doi: 10.1016/j.biopsych.2017.12.006.
(3) Misel ML, Gish RG, Patton H, Mendler M. Sodium benzoate for treatment of hepatic encephalopathy. Gastroenterol Hepatol (N Y). 2013 Apr;9(4):219-27.
(4) Piper JD, Piper PW. Benzoate and Sorbate Salts: A Systematic Review of the Potential Hazards of These Invaluable Preservatives and the Expanding Spectrum of Clinical Uses for Sodium Benzoate. Compr Rev Food Sci Food Saf. 2017 Sep;16(5):868-880. doi: 10.1111/1541-4337.12284.
(5) Nair B. Final report on the safety assessment of Benzyl Alcohol, Benzoic Acid, and Sodium Benzoate. Int J Toxicol. 2001;20 Suppl 3:23-50. doi: 10.1080/10915810152630729.
Abstract. Benzyl Alcohol is an aromatic alcohol used in a wide variety of cosmetic formulations as a fragrance component, preservative, solvent, and viscosity-decreasing agent. Benzoic Acid is an aromatic acid used in a wide variety of cosmetics as a pH adjuster and preservative. Sodium Benzoate is the sodium salt of Benzoic Acid used as a preservative, also in a wide range of cosmetic product types. Benzyl Alcohol is metabolized to Benzoic Acid, which reacts with glycine and excreted as hippuric acid in the human body. Acceptable daily intakes were established by the World Health Organization at 5 mg/kg for Benzyl Alcohol, Benzoic Acid, and Sodium Benzoate. Benzoic Acid and Sodium Benzoate are generally recognized as safe in foods according to the U.S. Food and Drug Administration. No adverse effects of Benzyl Alcohol were seen in chronic exposure animal studies using rats and mice. Effects of Benzoic Acid and Sodium Benzoate in chronic exposure animal studies were limited to reduced feed intake and reduced growth. Some differences between control and Benzyl Alcohol-treated populations were noted in one reproductive toxicity study using mice, but these were limited to lower maternal body weights and decreased mean litter weights. Another study also noted that fetal weight was decreased compared to controls, but a third study showed no differences between control and Benzyl Alcohol-treated groups. Benzoic Acid was associated with an increased number of resorptions and malformations in hamsters, but there were no reproductive or developmental toxicty findings in studies using mice and rats exposed to Sodium Benzoate, and, likewise, Benzoic Acid was negative in two rat studies. Genotoxicity tests for these ingredients were mostly negative, but there were some assays that were positive. Carcinogenicity studies, however, were negative. Clinical data indicated that these ingredients can produce nonimmunologic contact urticaria and nonimmunologic immediate contact reactions, characterized by the appearance of wheals, erythema, and pruritus. In one study, 5% Benzyl Alcohol elicited a reaction, and in another study, 2% Benzoic Acid did likewise. Benzyl Alcohol, however, was not a sensitizer at 10%, nor was Benzoic Acid a sensitizer at 2%. Recognizing that the nonimmunologic reactions are strictly cutaneous, likely involving a cholinergic mechanism, it was concluded that these ingredients could be used safely at concentrations up to 5%, but that manufacturers should consider the nonimmunologic phenomena when using these ingredients in cosmetic formulations designed for infants and children. Additionally, Benzyl Alcohol was considered safe up to 10% for use in hair dyes. The limited body exposure, the duration of use, and the frequency of use were considered in concluding that the nonimmunologic reactions would not be a concern. Because of the wide variety of product types in which these ingredients may be used, it is likely that inhalation may be a route of exposure. The available safety tests are not considered sufficient to support the safety of these ingredients in formulations where inhalation is a route of exposure. Inhalation toxicity data are needed to complete the safety assessment of these ingredients where inhalation can occur.
| Evaluate |

