| "Descrizione" by Nat45 (5724 pt) | 2023-Feb-27 12:37 |
Review Consensus: 7 Rating: 7 Number of users: 1
| Evaluation | N. Experts | Evaluation | N. Experts |
|---|---|---|---|
| 1 | 6 | ||
| 2 | 7 | ||
| 3 | 8 | ||
| 4 | 9 | ||
| 5 | 10 |
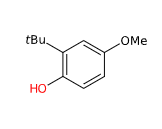
Butylated hydroxyanisole is a chemical compound of 2- and 3-tert-butyl-4-methoxyphenols.
It appears in form of white or yellowish powder soluble in alcohol propylene glycol. Insoluble in water.

What it is used for and where
Cosmetics
Butylhydroxyanisole or BHA, is a preservative, an antioxidant additive frequently found in toothpastes, liquid soaps and other personal care products.
Food
Widely used in the cosmetics industry. and in the food industry, especially in cereals, chewing gum, crisps, vegetable oils, also in pharmaceuticals, rubber and petroleum products
In foodstuffs to be used: only in fats and oils for professional preparation of heat-treated foodstuffs; frying oil and frying fat (excluding olive pomace oil) as well as lard, lard, fish oil, beef fat, poultry fat and sheep fat.
Labelled with the number E320 in the list of European food additives as an antioxidant.
Animal feed
Antioxidant in the diet of all animal species and categories.
Safety
The Panel on Food Additives and Nutrient Sources (EFSA, 2012 ) suggested for the acceptable daily intake (ADI) level is 1 mg/kg bw/day for BHA.
The Panel on Additives and Products or Substances used in Animal Feed (FEEDAP) considered BHA up to 150 mg/kg complete feed as safe for all animal species except for cats, for which a safe dose could not be established from the tolerance data. BHA is rapidly absorbed from the gastrointestinal tract; it is metabolised rapidly and excreted as such and as metabolites in the urine and faeces. The proportions of the different metabolites vary depending on species and dose. No accumulation of BHA or metabolites was observed in tissues. The Panel concluded that no concern for consumer safety would arise from the use of BHA as a feed additive at the maximum concentration of 150 mg/kg feed. The additive should be considered a skin, eye irritant and a potential skin sensitiser. Exposure of the user via inhalation was considered unlikely; therefore, a risk is not expected. The use of BHA at the maximum concentration proposed is unlikely to pose a risk to the environment. BHA is authorised as an antioxidant for food use at comparable use levels, therefore, no studies were required to demonstrate the efficacy of BHA as an antioxidant in feedingstuffs for all animal species (1).
Results of this study may support the safety of BHA, but also demonstrate the importance of performing toxicity evaluation at the cellular level besides the tissue level (2).
This study finds it difficult to attribute the endocrine disrupting effect to BHA (3).
The most relevant studies and their abstracts have been selected to explore this in more depth:
Butylated hydroxyanisole studies
Typical commercial product characteristics BHA Butylated hydroxyanisole
| Appearance | White or yellowish powder |
| Boiling Point | 264-270ºC |
| Melting Point | 48-63ºC |
| Flash Point | 130ºC |
| Vapor density | 6.2 |
| Residue on ignition | ≤ 0.05% |
| Phenolic impurities | ≤ 0.5% |
| Arsenic | ≤ 2 ppm |
| Heavy metal | ≤ 10 ppm |
| Mercury | ≤ 1 ppm |
| Lead | ≤ 2 ppm |
| Hydroquinone | ≤ 2% |
| Safety |  |
 |  |
 | 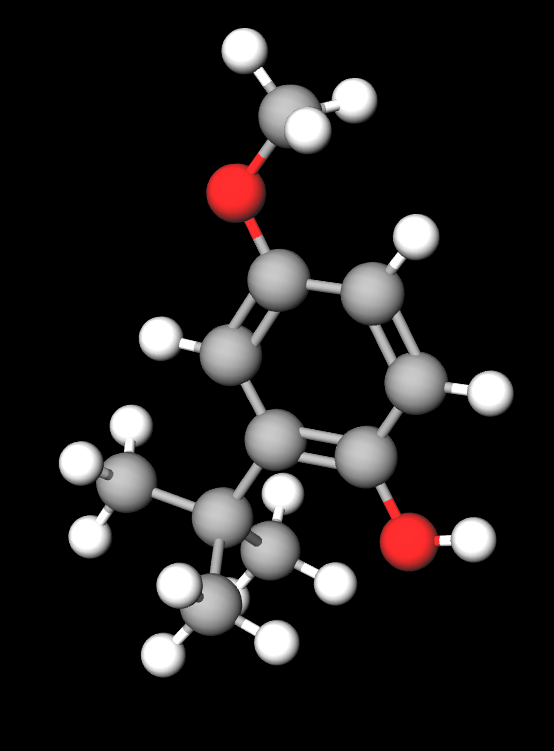 |
- Molecular Formula C11H16O2
- Linear Formula (CH3)3CC6H3(OCH3)OH
- Molecular Weight 360.494 g/mol
- Exact Mass 360.230072
- CAS : 25013-16-5 121-00-6 921-00-6
- EINECS 246-563-8
- UNII 62RAC24292
- DSSTox Substance ID DTXSID7040788
- IUPAC 2-tert-butyl-4-methoxyphenol
- InChI=1S/C11H16O2/c1-11(2,3)9-7-8(13-4)5-6-10(9)12/h5-7,12H,1-4H3
- InChl Key MRBKEAMVRSLQPH-UHFFFAOYSA-N
- SMILES CC(C)(C)C1=C(C=CC(=C1)OC)O
- MDL number MFCD01779059
- PubChem Substance ID
- ChEBI 76358
- FEMA 2183
- NACRES NA.22
Synonyms :
- Butylhydroxyanisole
- BHA
- 2-tert-Butyl-4-methoxyphenol
- Hydroxyanisole, Butylated
- 2-(1,1-dimethylethyl)-4-methoxyphenol
- 2-Butyl-4-hydroxyanisole
- 2-(tert-butyl)-4-methoxyphenol
- 4-Methoxy-2-tert-butylphenol
- Phenol, 2-tert-butyl-4-methoxy-
- 2-(1,1-Dimethylethyl)-4-methoxy-phenol
- 4-Methoxy-6-tert-butylphenol
- 3-tert-Butyl-p-hydroxyanisole
- 2-tert-Butyl-4-methoxy-phenol
References_____________________________________________________________
(1) EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP), Rychen G, Aquilina G, Azimonti G, Bampidis V, Bastos ML, Bories G, Chesson A, Cocconcelli PS, Flachowsky G, Kolar B, Kouba M, López-Alonso M, Puente SL, Mantovani A, Mayo B, Ramos F, Saarela M, Villa RE, Wallace RJ, Wester P, Lundebye AK, Nebbia C, Renshaw D, Innocenti ML, Gropp J. Safety and efficacy of butylated hydroxyanisole (BHA) as a feed additive for all animal species. EFSA J. 2018 Mar 28;16(3):e05215. doi: 10.2903/j.efsa.2018.5215.
(2) Mizobuchi M, Ishidoh K, Kamemura N. A comparison of cell death mechanisms of antioxidants, butylated hydroxyanisole and butylated hydroxytoluene. Drug Chem Toxicol. 2021 May 20:1-8. doi: 10.1080/01480545.2021.1894701.
(3) Pop A, Kiss B, Loghin F. Endocrine disrupting effects of butylated hydroxyanisole (BHA - E320). Clujul Med. 2013;86(1):16-20.
| Evaluate |

