| "Descrizione" by A_Partyns (12948 pt) | 2023-Jun-15 18:08 |
Review Consensus: 10 Rating: 10 Number of users: 1
| Evaluation | N. Experts | Evaluation | N. Experts |
|---|---|---|---|
| 1 | 6 | ||
| 2 | 7 | ||
| 3 | 8 | ||
| 4 | 9 | ||
| 5 | 10 |
Methylisothiazolinone (2-methyl-1,2-thiazol-3-one or 2-Methyl-4-Isothiazolin-3-one) is a chemical compound that includes a 1,2-thazole which is a 4-isothiazolin-3-one with a methyl group on the nitrogen atom.
The synthesised extraction process takes place in three steps:
- Isothiazolin ring formation. The first step is the reaction of an amine, such as ammonia or a primary or secondary amine, with carbon disulphide in a process known as the Willgerodt-Kindler reaction. A dithiocarbamate salt is thus formed.
- Oxidation. The dithiocarbamate salt is oxidised using hydrogen peroxide or another suitable oxidising agent. An isothiazolinone ring is thus formed.
- Methylation. In the third and final step a methyl group is attached to the nitrogen atom in the isothiazolinone ring through a process known as methylation. Methyl isothiazolinone is thus formed.
It occurs in liquid form with a slightly yellow color or as a colorless liquid depending on concentration

What it is used for and where
Methylisothiazolinone is a biocide, i.e. it can kill microorganisms. It is often used as a preservative in products such as paints, coatings and personal care products to prevent the growth of bacteria and fungi. It is effective at very low concentrations and is active against a wide range of microorganisms. However, it can cause allergic reactions in some people, so its use is regulated in many countries.
Cosmetics
Preservative. Any product containing organic, inorganic compounds, water, needs to be preserved from microbial contamination. Preservatives act against the development of harmful microorganisms and against oxidation of the product.
It is a restricted ingredient V/57, V/39 as a Relevant Item in the Annexes of the European Cosmetics Regulation 1223/2009. Maximum concentration in the ready-for-use preparation 0.0015 % in rinse products.
It acts as a preservative, antioxidant, bactericide and biocide commonly used by the cosmetic industry in liquid soaps, shampoos and the paint industry. As a bactericide, it is used in industrial water and wastewater treatment to eliminate algae, fungus, bacteria. And also in industrial washing products, in waste water from oil wells, in paper and rubber manufacturing.
In 2005 EU legislation allowed the use of this component, which is usually together with Methylchloroisothiazolinone, at concentrations up to 100 ppm, a measure 25 times higher than previously allowed, and there has been an unprecedented global increase in allergic contact dermatitis (1).
Cosmetic Safety
Accredited studies have shown that MIT is a human sensitizer, to the extent that MIT was named allergen of the year in 2013 (2).
Many cases of allergy have been established, and there is a wealth of scientific documentation on the subject (3).
In light of the exceptionally high cases of contact allergy to the preservative methylisothiazolinone, this study deemed it necessary to learn about the reactivity between methylisothiazolinone, , octylisothiazolinone, and benzisothiazolinone. The results indicate that individuals sensitized to methylisothiazolinone, may react to octylisothiazolinone and benzisothiazolinone when exposed to sufficient concentrations (4).
"Methylisothiazolinone, studies"
Typical optimal characteristics of the commercial product Methylisothiazolinone
| Appearance | Slight yellow liquid o transparent liquid |
| Total actives Contents | 14.0-15.0% |
| Chlorine | 3 – 5% |
| pH Value | 1.0-5.0 |
| Specific gravity/25°C | 1.26-1.32 |
| Melting point | 254-256 °C(lit.) |
| Boiling point | 182.8±23.0 °C at 760 mmHg |
| Flash Point | 64.3±22.6 °C |
| PSA | 50.24000 |
| Vapour Pressure | 0.8±0.3 mmHg at 25°C |
| LogP | 0.05 |
| Index of Refraction | 1.589 |
| Safety |  |
 |  |
 |  |
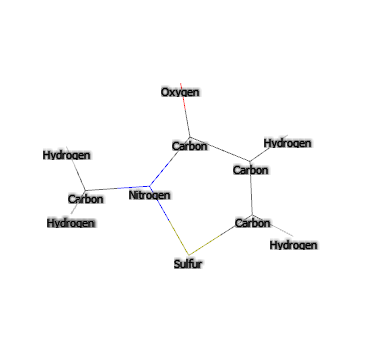
- Molecular Formula : C4H5NOS
- Molecular Weight : 115.15
- CAS : 2682-20-4
- UNII 229D0E1QFA
- EC Number: 220-239-6
- DSSTox Substance ID: DTXSID2034259
- MDL number MFCD01742315
- PubChem Substance ID 329764182
- InChI=1S/C4H5NOS/c1-5-4(6)2-3-7-5/h2-3H,1H3
- InChl Key BEGLCMHJXHIJLR-UHFFFAOYSA-N
- SMILES CN1C(=O)C=CS1
- IUPAC 2-methyl-1,2-thiazol-3-one
- ChEBI 53620
- Beilstein 606203
- NACRES NA.21
Synonyms :
- 2-Methyl-4-isothiazolin-3-one
- 2-Methyl-3(2H)-isothiazolone
- 2-methyl-4-isothiazolin-3-one MIT
- 2-Methylisothiazol-3(2H)-one
- 2-Methylisothiazole-3-one
- Beilstein Registry Number: 606203
- EC Number: 220-239-6
- MDL number: MFCD01742315
- PubChem Substance ID 329765047
References_________________________________________________________________________
(1) Venables ZC, Bourke JF, Buckley DA, Campbell F, Chowdhury MMU, Abdul-Ghaffar S, Green C, Holden CR, McFadden J, Orton D, Sabroe RA, Sansom J, Stone NM, Wakelin SH, Wilkinson SM, Johnston GA. Has the epidemic of allergic contact dermatitis due to methylisothiazolinone reached its peak? Br J Dermatol. 2017 Jul;177(1):276-278. doi: 10.1111/bjd.15016.
(2) Castanedo-Tardana MP, Zug KA. Methylisothiazolinone. Dermatitis. 2013 Jan-Feb;24(1):2-6. doi: 10.1097/DER.0b013e31827edc73.
Van Huizen AV, Tseng AS, Beane WS. Methylisothiazolinone toxicity and inhibition of wound healing and regeneration in planaria. Aquat Toxicol. 2017 Oct;191:226-235. doi: 10.1016/j.aquatox.2017.08.013.
(3) Todberg T, Opstrup MS, Johansen JD, Hald M. Occupational facial contact dermatitis caused by methylchloroisothiazolinone/methylisothiazolinone in a stainless steel aerosol spray. Contact Dermatitis. 2017 Sep;77(3):173-174. doi: 10.1111/cod.12773.
Silva CA, El-Houri RB, Christensen LP, Andersen F. Contact allergy caused by methylisothiazolinone in shoe glue. Contact Dermatitis. 2017 Sep;77(3):175-176. doi: 10.1111/cod.12785.
(4) Goodier MC, Ljungberg L, Persson C, Engfeldt M, Bruze M, Warshaw EM. Allergic Contact Dermatitis From Methylisothiazolinone in Residential Wall Paint. Dermatitis. 2017 Jul/Aug;28(4):284-287. doi: 10.1097/DER.0000000000000294.
| Evaluate |

