| "Descrizione" by A_Partyns (12948 pt) | 2024-Sep-20 19:09 |
Review Consensus: 7 Rating: 7 Number of users: 1
| Evaluation | N. Experts | Evaluation | N. Experts |
|---|---|---|---|
| 1 | 6 | ||
| 2 | 7 | ||
| 3 | 8 | ||
| 4 | 9 | ||
| 5 | 10 |
Linalool (3,7-dimethyl-1,6-octadien-3-ol) is a monoterpene alcohol that is extracted from aromatic plants such as Lavandula sp., Ocinum Basilicum L. and other plants of the Lamiaceae and Rutaceae families. It is also extracted by organic synthesis from α, β-pinene or other terpenes and isolated from Plantaginaceae, rosewood oil, carrageenan oil, coriander oil and eucalyptus oil. Linalool is oxidised to form Citral, a substance that can also be used to chemically produce many other flavourings.
The name describes the structure of the molecule:
- Linal is derived from 'linaloe', which is a type of wood from trees belonging to the Burseraceae family. Linalool was first isolated from linaloe oil, hence the name.
- ool is a suffix used in organic chemistry to indicate substances that are alcohols. In the case of linalool, it refers to the presence of a hydroxyl functional group (-OH) in the molecule.
Natural chemical synthesis:
- Linalool is produced naturally by plants during photosynthesis. It is synthesised as part of plant metabolism and is responsible for emitting their characteristic aroma.
The industrial synthesis process takes place in different steps:
- Raw material extraction. Linalool is extracted from plants that produce it in high quantities, such as lavender, mint and coriander. The plant material is collected and prepared for extraction.
- Distillation. The plant material undergoes steam distillation, a process in which steam causes the plant cells to break down and release the oils, including linalool.
- Condensation and separation. The mixture of steam and oil is cooled and condensed and the resulting liquid is a mixture of water and oil, which can be separated because oil and water do not mix. The oil layer, which contains linalool, is collected.
- Purification. The oil is further purified to isolate the linalool by various methods, including fractional distillation, in which the oil is heated and the components that vaporise at different temperatures are separated.
- Quality control. Linalool is tested to ensure that it meets the necessary quality standards.
Linalool occurs as a clear, colourless to pale yellow liquid, relatively soluble in water and highly soluble in many organic solvents.

What it is used for and where it is used
Medicine
Due to its high penetration potential and its affinity for cell membranes, it has the ability to bind to various molecular structures such as protein glycoproteins. It exists in two enantiomers with different pharmacological effects: (R)-linalool works as an anti-stress agent, (S)-linalool increases heart rate.
It is known to have anti-inflammatory, anti-cancer, anti-hyperlipidaemia, antibacterial and neuroprotective properties (1).
Food
It is an ingredient approved by the Food and Drug Administration (FDA) as GRAS so it is recognised as a safe food additive and is included to impart fragrance to food products in which it is incorporated. It is frequently used as a flavouring in butter, grapes, apricots, pineapples. Oxidation must be avoided.
Cosmetics
It is a chemical intermediate component. Fragrance included in toothpastes, sunscreens, lipsticks, deodorants, lip balms. It is a component that is considered safe if it is not oxidised, so remember to close the cap of the package in which it is inserted tightly to prevent air from penetrating inside for a long time and causing oxidation not only of this, but also of any other oxidation-sensitive components.
Deodorant agent. When substances that give off an unpleasant odour are included in cosmetic formulations (typical examples are methyl mercaptan and hydrogen sulphide derived from garlic), deodorants attenuate or eliminate the unpleasant exhalation.
Perfuming. Unlike fragrance, which can also contain slightly less pleasant or characteristic odours, the term perfume indicates only very pleasant fragrances. Used for perfumes and aromatic raw materials.
Pharmaceutical
Fragrance in various drug formulations e.g. anti-caries.
Other uses
Tobacco, insecticides
The most relevant studies and their abstracts have been selected to explore this in more depth:
Typical optimal commercial product characteristics Linalool
| Appearance | Clear colourless to pale yellow liquid |
| Boiling point | 194-197 °C/720 mmHg (lit.) |
| Melting Point | 25°C |
| Density | 0.87 g/mL at 25 °C (lit.) 0.9±0.1 g/cm3 |
| Flash Point | 76.1±0.0 °C |
| Vapour Pressure | 0.1±0.8 mmHg at 25°C |
| pH | 4.5 (1.45g/l, H2O, 25℃) |
| pka | 14.51±0.29 |
| Specific Gravity | 0.860 (20/4℃) |
| Explosive Limit | 0.9-5.2%(V) |
| Water Solubility | 1.45 g/L (25ºC) |
| PSA | 20.23000 |
| LogP | 3.28 |
| Storage | 2-8°C |
| Safety |  |
 |  |
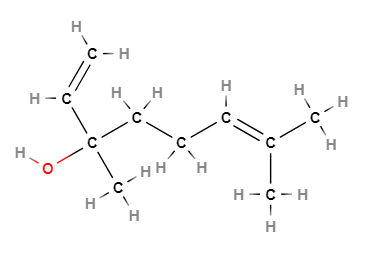 |  |
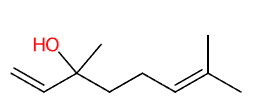
- Molecular Formula C10H18O (CH3)2C=CH(CH2)2C(CH3)(OH)CH=CH2
- Molecular Weight 154.25
- Exact Mass 154.135757
- CAS 78-70-6
- UNII D81QY6I88E
- EC Number 201-134-4
- DSSTox Substance ID DTXSID7025502
- IUPAC 3,7-dimethylocta-1,6-dien-3-ol
- InChI=1S/C10H18O/c1-5-10(4,11)8-6-7-9(2)3/h5,7,11H,1,6,8H2,2-4H3
- InChl Key CDOSHBSSFJOMGT-UHFFFAOYSA-N
- SMILES CC(=CCCC(C)(C=C)O)C
- MDL number MFCD00008906
- PubChem Substance ID 24896318
- Beilstein 1721488
- NACRES NA.22
- ChEBI 17580
- ICSC 0912
- JECFA 356
- FEMA 2635
- RTECS RG5775000
Synonyms :
- 3,7-Dimethylocta-1,6-dien-3-ol
- Linalol
- 1,6-Octadien-3-ol, 3,7-dimethyl-
- 2,6-Dimethyl-2,7-octadien-6-ol
- 3,7-dimethyl-octa-1,6-dien-3-ol
- 2,7-Octadien-6-ol, 2,6-dimethyl-
- (1)-3,7-Dimethyl-1,6-octadien-3-ol
- dl-3,7-Dimethyl-3-hydroxy-1,6-octadiene
References_________________________________________________________________________
(1) Pereira I, Severino P, Santos AC, Silva AM, Souto EB. Linalool bioactive properties and potential applicability in drug delivery systems. Colloids Surf B Biointerfaces. 2018 Nov 1;171:566-578. doi: 10.1016/j.colsurfb.2018.08.001.
An Q, Ren JN, Li X, Fan G, Qu SS, Song Y, Li Y, Pan SY. Recent updates on bioactive properties of linalool. Food Funct. 2021 Nov 1;12(21):10370-10389. doi: 10.1039/d1fo02120f. PMID: 34611674.
Abstract. Natural products, including essential oils and their components, have been used for their bioactivities. Linalool (2,6-dimethyl-2,7-octadien-6-ol) is an aromatic monoterpene alcohol that is widely found in essential oils and is broadly used in perfumes, cosmetics, household cleaners and food additives. This review covers the sources, physicochemical properties, application, synthesis and bioactivities of linalool. The present study focuses on the bioactive properties of linalool, including anticancer, antimicrobial, neuroprotective, anxiolytic, antidepressant, anti-stress, hepatoprotective, renal protective, and lung protective activity and the underlying mechanisms. Besides this, the therapeutic potential of linalool and the prospect of encapsulating linalool are also discussed. Linalool can induce apoptosis of cancer cells via oxidative stress, and at the same time protects normal cells. Linalool exerts antimicrobial effects through disruption of cell membranes. The protective effects of linalool to the liver, kidney and lung are owing to its anti-inflammatory activity. On account of its protective effects and low toxicity, linalool can be used as an adjuvant of anticancer drugs or antibiotics. Therefore, linalool has a great potential to be applied as a natural and safe alternative therapeutic.
Singh S, Mishra A. Linalool: Therapeutic Indication And Their Multifaceted Biomedical Applications. Drug Res (Stuttg). 2024 Jul;74(6):255-268. doi: 10.1055/a-2321-9571. Epub 2024 Jul 5. PMID: 38968949.
Abstract. This comprehensive review endeavors to illuminate the nuanced facets of linalool, a prominent monoterpene found abundantly in essential oils, constituting a massive portion of their composition. The biomedical relevance of linalool is a key focus, highlighting its therapeutic attributes observed through anti-nociceptive effects, anxiolytic properties, and behavioral modulation in individuals affected by dementia. These findings underscore the compound's potential application in biomedical applications. This review further explores contemporary formulations, delineating the adaptability of linalool in nano-emulsions, microemulsions, bio-capsules, and various topical formulations, including topical gels and lotions. This review covers published and granted patents between 2018-2024 and sheds light on the evolving landscape of linalool applications, revealing advancements in dermatological, anti-inflammatory, and antimicrobial domains. Thieme. All rights reserved.
Letizia CS, Cocchiara J, Lalko J, Api AM. Fragrance material review on linalool. Food Chem Toxicol. 2003 Jul;41(7):943-64. doi: 10.1016/s0278-6915(03)00015-2.
| Evaluate |

