| "Descrizione" by FRanier (9971 pt) | 2023-Dec-04 12:45 |
Review Consensus: 10 Rating: 10 Number of users: 1
| Evaluation | N. Experts | Evaluation | N. Experts |
|---|---|---|---|
| 1 | 6 | ||
| 2 | 7 | ||
| 3 | 8 | ||
| 4 | 9 | ||
| 5 | 10 |
Tetrasodium Pyrophosphate is a chemical component used in various industries, including food production, dental care, and detergent manufacturing.
The name defines the structure of the molecule:
- Tetrasodium indicates the presence of four sodium atoms in the compound. Sodium is a chemical element that, in this context, is bonded with other elements to form the compound.
- Pyrophosphate refers to a salt or ester of pyrophosphoric acid. Pyrophosphate consists of two phosphate units bonded together. It is known for its ability to bind heavy metals and for its emulsifying and stabilizing properties.
The synthesis process takes place in several steps:
- Preparation of raw materials. The raw materials for the synthesis of tetrasodium pyrophosphate are sodium carbonate and phosphoric acid.
- Chemical reaction. Sodium carbonate enters into a reaction with phosphoric acid to produce sodium phosphate, is then heated to produce sodium pyrophosphate, which is further reacted with sodium hydroxide to produce tetrasodium pyrophosphate.
- Crystallization and purification. The resulting solution is allowed to cool, causing the tetrasodium pyrophosphate to crystallize from the solution. The crystals are then collected and washed to remove any impurities.
- Drying and packaging. The purified tetrasodium pyrophosphate is dried and ready for marketing.
It comes in the form of a white powder.

What it is for and where
Tetrasodium Pyrophosphate is commonly used as a leavening agent in baked goods, as a stabilizer in dairy products, and as a chelating agent in detergents and oral hygiene products. It also has applications in the ceramics industry and water treatment.
- water softener
- pH stabilizer or acidity factor
- metal cleaner
- dietary supplement
- emulsifier in cosmetics
- detergent in soaps
- detergent in wool washing
- anticalculus and antimicrobial in toothpastes
Medical
Pyrophosphate, sequestering calcium in the body, is a potent mitogen, capable of stimulating proliferation in multiple cell types, and a critical participant in bone mineralization (1).
The human oral cavity comprehends a very complex ecosystem which may harbor several species of microorganisms. Mechanical removal of oral biofilm, which includes tooth brushing, tongue scraping and flossing, are considered the most effective method of oral hygiene, significantly reducing microbial charge in the oral cavity. Regarding flosses containing anti-caries, gingivitis and antiplaque agents, there is an increasing range of products with highly efficient physical and chemical components. Texturized dental floss made from nylon and entangled floss made from polypropylene could be commonly found for oral hygiene; both may have antimicrobial agents (such as triclosan, chlorhexidine and tetrasodium pyrophosphate) added to reduce the microbial colonization in the subgingival sulcus and in the interproximal spaces (1).
Some toothpastes which contain in their compositions anticalculus agents such as pyrophosphate, may provide reduction in biofilm formation by approximately 15% when compared to toothpastes without this active principle (2).
Food
Tetrasodium pyrophosphate (TSP) is used in processed meat products, as an emulsifier in cheese, and as a color preservative in soybean paste. Based on the results, TSP is unclassified according to the Globally Harmonization System, with an LD(50) value of over 2,000 mg/kg. The no observed effect level (NOEL) and no observed adverse effect level (NOAEL) were 250 and 500 mg/kg /day respectively and the target organ appears to be the kidney (3).
Cosmetics
Anticaking agent. This compound facilitates free flow and prevents aggregation or clumping of substances in a formulation by reducing the tendency of certain particles to stick together.
Buffering agent. It is an iingredient that can bring an alkaline or acid solution to a certain pH level and prevent it from changing, in practice a pH stabiliser that can effectively resist instability and pH change.
Chelating agent. It has the function of preventing unstable reactions and improving the bioavailability of chemical components within a product, and removes calcium and magnesium cations that can cause cloudiness in clear liquids.
Oral care agent. This ingredient can be placed in the oral cavity to improve and/or maintain oral hygiene and health, to prevent or improve a disorder of the teeth, gums, mucous membrane. It provides cosmetic effects to the oral cavity as a protector, cleanser, deodorant.
Commercial applications
Food industry. Used as a leavening agent in baked goods.
Gelatin production. Helps in improving the texture and stability of gelatins.
Dental products. Added to toothpaste as a tartar control agent.
Detergents. Used in cleaning agents for its ability to remove and suspend dirt.
Ceramics industry. Employed in ceramic production to improve physical properties.
Medical Applications
Tissue oxygenation. In some instances, it may be used as a chelating agent to facilitate tissue oxygenation.
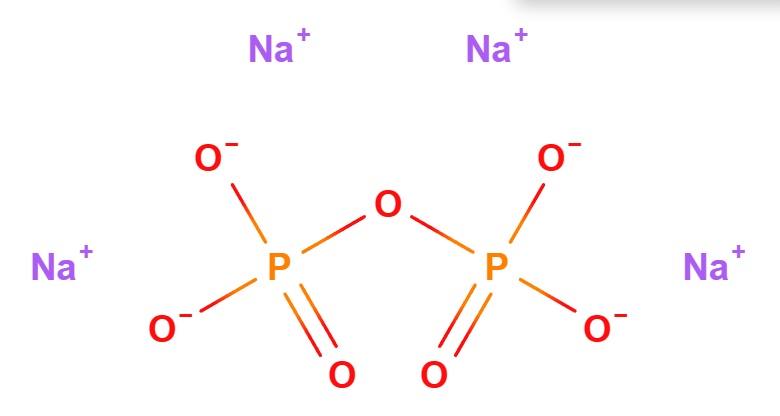 |  |
- Molecular Formula Na4O7P2
- Molecular Weight 265.9
- CAS 7722-88-5
- EC number 231-767-1
Synonyms:
- Sodium pyrophosphate
- TSPP
- Tetrasodium diphosphate
References_______________________________________
(1) Pujari-Palmer M, Pujari-Palmer S, Lu X, Lind T, Melhus H, Engstrand T, Karlsson-Ott M, Engqvist H. Pyrophosphate Stimulates Differentiation, Matrix Gene Expression and Alkaline Phosphatase Activity in Osteoblasts. PLoS One. 2016 Oct 4;11(10):e0163530. doi:10.1371/journal.pone.0163530.
Abstract. Pyrophosphate is a potent mitogen, capable of stimulating proliferation in multiple cell types, and a critical participant in bone mineralization. Pyrophosphate can also affect the resorption rate and bioactivity of orthopedic ceramics. The present study investigated whether calcium pyrophosphate affected proliferation, differentiation and gene expression in early (MC3T3 pre-osteoblast) and late stage (SAOS-2 osteosarcoma) osteoblasts. Pyrophosphate stimulated peak alkaline phosphatase activity by 50% and 150% at 100μM and 0.1μM in MC3T3, and by 40% in SAOS-2. The expression of differentiation markers collagen 1 (COL1), alkaline phosphatase (ALP), osteopontin (OPN), and osteocalcin (OCN) were increased by an average of 1.5, 2, 2 and 3 fold, by high concentrations of sodium pyrophosphate (100μM) after 7 days of exposure in MC3T3. COX-2 and ANK expression did not differ significantly from controls in either treatment group. Though both high and low concentrations of pyrophosphate stimulate ALP activity, only high concentrations (100μM) stimulated osteogenic gene expression. Pyrophosphate did not affect proliferation in either cell type. The results of this study confirm that chronic exposure to pyrophosphate exerts a physiological effect upon osteoblast differentiation and ALP activity, specifically by stimulating osteoblast differentiation markers and extracellular matrix gene expression.
(2) Pedrazzi V, Corsi LP, Pedrazzi H, Netto EI, Nascimento Cd, Issa JP. Clinical evaluation of residual tetrasodium pyrophosphate released from two different anticalculus flosses. Braz Dent J. 2015 Mar-Apr;26(2):116-20. doi: 10.1590/0103-6440201300093.
Abstract. The aim of this study was to compare the residual content of tetrasodium pyrophosphate released by two different anticalculus dental flosses (Reach PP®--entangled polypropylene floss and Reach NT®--texturized nylon) in the oral cavity. Ten healthy individuals (aged between 18 and 30 years) were enrolled in this randomized crossover clinical investigation. Participants received instructions on daily dental flossing and the interventions were randomly performed in 2 different groups (NT or PP) of five individuals each according to the dental flosses. Individuals were instructed to use each dental floss with a total of six slides on the two interproximal aspects of target teeth (3 slides on each interproximal aspect). A washout period of one week was used before start flossing interventions and after each type of dental floss to prevent any bias related to the exposure to any product that contained the active ingredient. Samples were collected by #35 sterilized absorbent paper points from interdental fluid after flossing and assessed by ion chromatography. The levels of residual tetrasodium pyrophosphate were evaluated by means of binomial generalized linear model proportions and canonical link function. Both dental flosses were effective in tetrasodium pyrophosphate release at therapeutic levels in the interdental gingival crevicular fluid for a period of up to 2 h after use. No significant differences were found between both groups (p>0.05). It may be concluded that both material composition and physical structure of the new dental floss did not affect the release or the maintenance of anticalculus agent at therapeutic levels for a period of up to 2 h after single use.
(3) Grossman E, Hou L, Bollmer BW, Court LK, Mcclary JM, Bennett S, et al.. Triclosan/pyrophosphate dentifrice: dental plaque and gingivitis effects in a 6 month randomized controlled clinical study. J Clin Dent 2002;13:149-157.
Abstract. A double-blind, parallel, randomized and controlled clinical trial was conducted on 186 subjects over six months to assess the effects of a 0.28% triclosan/5% pyrophosphate (with NaF/silica) dentifrice on dental plaque and gingivitis as compared to a NaF/silica negative control dentifrice. An initial examination was performed to assess the health of the oral soft and hard tissues and to measure plaque (by Turesky modified Quigley-Hein Plaque Index), gingivitis (by Löe-Silness Gingival and Ainamo and Bay Gingival Bleeding [GBI] indices). Only those subjects with a GBI score > or = 5 were accepted into the study. Each enrolled subject received an oral prophylaxis and was requested to brush and floss twice per day with the negative control NaF/silica dentifrice. After one month, the subjects were recalled and a baseline examination was performed for each of the previously described parameters. Following the baseline examination, the subjects received another oral prophylaxis. The subjects were then separated by gender and by baseline GBI scores of < or = 7 or > 7 and arrayed by the changes in GBI bleeding sites from initial to baseline. Within strata, subjects were randomly assigned to brush twice per day with either the triclosan/pyrophosphate dentifrice or the negative control dentifrice. The subjects were subsequently examined for all of the above-described parameters following use of the test dentifrices for five weeks, three and six months. The data generated in this trial were analyzed using an analysis of covariance on all indices for all subjects completing the examinations. The results from this study demonstrated that the use of the triclosan/pyrophosphate dentifrice resulted in statistically significant reductions of dental plaque compared to the control by 10% (p < 0.05), 15.4% (p < 0.01) and 13.9% (p < 0.01) at five weeks, three and six months, respectively. However, there were no statistically significant differences between the test dentifrices for any of the gingivitis or gingival bleeding evaluations throughout the study. Based on 1) the fact that subjects possessed plaque-induced gingivitis in this clinical study, 2) the similarity in the magnitude of the plaque reductions observed from the triclosan/pyrophosphate dentifrice relative to those reported for other triclosan-containing dentifrices, 3) the similarity in the dose of triclosan relative to other triclosan dentifrices, and 4) the reported magnitude of gingivitis reductions from other triclosan-containing dentifrices, these findings were unexpected. Possible explanations of these results are that the triclosan/pyrophosphate dentifrice may be uniquely different from other triclosan dentifrices relative to its effects on gingivitis, or alternatively, the clinical design utilized here may not be optimized for triclosan/pyrophosphate dentifrice.
(4) Dong Seok Seo, Min Kwon, Ha Jung Sung, Cheol Beom Park Acute Oral or Dermal and Repeated Dose 90-Day Oral Toxicity of Tetrasodium Pyrophosphate in Spraque Dawley (SD) Rats Environ Health Toxicol. 2011;26:e2011014. doi: 10.5620/eht.2011.26.e2011014.
Abstract. Objectives: Tetrasodium pyrophosphate (TSP) is used in processed meat products, as an emulsifier in cheese, and as a color preservative in soybean paste. However, little is known about its toxicity. This study was conducted to investigate the potential acute and repeated dose toxicity of TSP in Spraque Dawley (SD) rats.....Conclusions: Based on the results, TSP is unclassified according to the Globally Harmonization System, with an LD(50) value of over 2,000 mg/kg. The no observed effect level (NOEL) and no observed adverse effect level (NOAEL) were 250 and 500 mg/kg /day respectively and the target organ appears to be the kidney.
| Evaluate |

