| "Descrizione" by A_Partyns (12948 pt) | 2023-Jun-26 09:45 |
Review Consensus: 10 Rating: 10 Number of users: 1
| Evaluation | N. Experts | Evaluation | N. Experts |
|---|---|---|---|
| 1 | 6 | ||
| 2 | 7 | ||
| 3 | 8 | ||
| 4 | 9 | ||
| 5 | 10 |
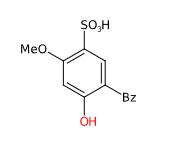
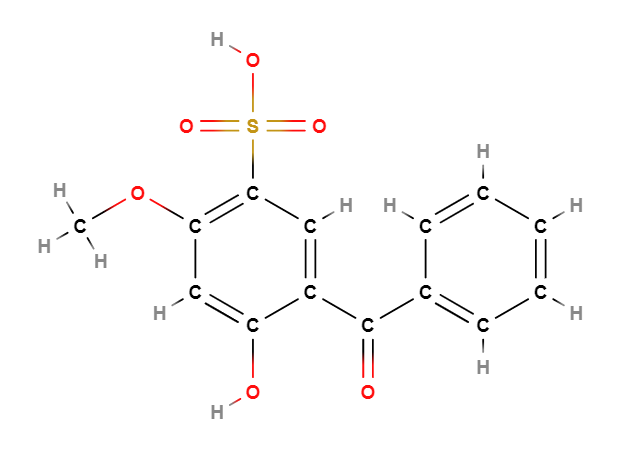
Benzophenone-4 (Sulisobenzone) is a chemical compound, sodium salt of benzenesulfonic acid.
The name describes the structure of the molecule:
- Benzene is a simple aromatic ring (a hexagonal ring of carbon atoms) with single and double alternating bonds. It is one of the fundamental structures in organic chemistry. Benzene is used in the production of a wide variety of chemicals and is also a natural component of crude oil.
- Phenone refers to a compound that contains a carbonyl group (C=O) attached to two carbon atoms, one of which is part of an aromatic ring. The carbonyl group is a functional group characterized by a double carbon-oxygen bond. Phenons are often used in organic chemistry as building blocks for more complex molecules.
- Sulfonyl group (SO2) is a functional group consisting of a sulfur atom bound to two oxygen atoms (double bonds) and a carbon atom of the benzene ring. Sulfonyl groups are often found in organic sulfur compounds, including some medications and dyes.
- Hydroxyl group (-OH) consists of an oxygen atom bound to a hydrogen atom. It is a common functional group in organic chemistry and is characteristic of alcohols. Hydroxyl groups can increase the polarity of organic molecules and improve their solubility in water.
- Second benzene ring is attached to the sulfonyl group. As mentioned above, benzene is a fundamental structure in organic chemistry and is used in the production of a wide variety of chemicals.
The synthesis process takes place in different steps:
- Preparation of benzophenone. Benzophenone can be synthesized from benzene Friedel-Crafts acylation with benzoyl chloride in the presence of Lewis acid typically aluminum chloride (AlCl3).
- Sulfonation of benzophenone. Benzophenone is sulfonated using sulfuric acid (H2SO4) to form benzophenone-4-sulfonic acid.
- Neutralization. Benzophenone-4-sulfonic acid is then neutralized with a base such as sodium hydroxide (NaOH) to form the sodium salt, which is benzophenone-4.
It appears in the form of a white powder

What it is used for and where
Cosmetics
It is widely used as a UV filter in sunscreens but is a restricted ingredient as VI/22 a Relevant Item in the Annexes of the European Cosmetics Regulation 1223/2009.
It has the following INCI functions:
Light stabilizer. It prevents light from degrading light-sensitive components and slows down degradation reactions that have already begun. The mechanism is, in a way, similar to antioxidants and the effectiveness depends on the.complexity of the formulation and the density of the product.
UV absorber. It acts by intercepting ultraviolet light before it can cause damage by reducing its energy through dissipation and returning it to a lower energy state.
UV filter. It is the defining ingredient in sun creams that can mitigate the sun's ultraviolet (UV) radiation, which is a high risk factor for the development of skin cancer, erythema and photo-ageing.
Other Uses
Moreover this substance is used in the following products:
- cosmetics and personal care products
- washing & cleaning products
- leather treatment products
- pharmaceuticals
- polishes and waxes
- coating products
- adhesives and sealants
- air care products
- plant protection products
- machine wash liquids/detergents
- automotive care products
- paints and coating or adhesives
- fragrances and air fresheners
| Appearance | White powder |
| Melting Point | 170°C |
| Density | 1.4±0.1 g/cm3 |
| PSA | 109.28000 |
| Refraction Index | 1.617 |
| LogP | 0.89 |
| Storage | |
| Safety |  |
 |  |
 |  |
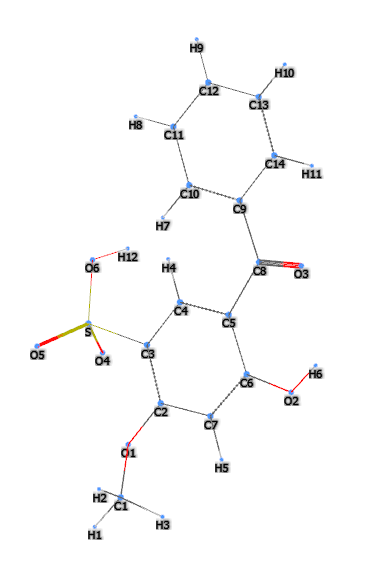
- Formula molecolare: C14H12O6S
- Peso molecolare: 308.304 g/mol
- Massa esatta 308.035461
- UNII: 1W6L629B4K
- CAS: 4065-45-6 6628-37-1 62121-63-5
- EC Number: 223-772-2 613-918-7
- PubChem Substance ID 329757575
- MDL number MFCD00024962
- Beilstein Registry Number 2889165
- DSSTox Substance ID
- IUPAC 5-benzoyl-4-hydroxy-2-methoxybenzenesulfonic acid
- InChI=1S/C14H12O6S/c1-20-12-8-11(15)10(7-13(12)21(17,18)19)14(16)9-5-3-2-4-6-9/h2-8,15H,1H3,(H,17,18,19)
- InChl Key CXVGEDCSTKKODG-UHFFFAOYSA-N
- SMILES COC1=C(C=C(C(=C1)O)C(=O)C2=CC=CC=C2)S(=O)(=O)O
- ChEBI 135312
- RTECS DB5044300
Synonyms:
- Sulisobenzone
- 5-benzoyl-4-hydroxy-2-methoxy-benzenesulfonic acid
- 4-hydroxy-2-methoxy-5-(phenylcarbonyl)benzenesulfonic acid
- Benzophenone-4
- 5-Benzoyl-4-hydroxy-2-methoxybenzolsulfonsaeure
- 5-Benzoyl-4-hydroxy-2-methoxybenzene sulfonic acid
- 1-Phenol-4-sulfonic acid, 2-benzoyl-5-methoxy-
- 5-Benzoyl-4-hydroxy-2-methoxybenzenesulfonic acid ammoniate
- 5-Benzoyl-4-hydroxy-2-methoxybenzenesulfonic acid
- 2-Hydroxy-4-methoxybenzophenone-5-sulfonic acid
- 1-Phenol-4-sulfonic acid, 2-benzoyl-5-methoxy-, sodium salt
- 4-hydroxy-2-methoxy-5-(oxo-phenylmethyl)benzenesulfonic acid
- Benzenesulfonic acid, 5-benzoyl-4-hydroxy-2-methoxy-, sodium salt
- SCHEMBL16330
- 2-Hydroxy-4-Methoxy-5-sulfonylbenzophenone(BP-4)
- 1-Phenol-4-sulfonic acid, 2-benzoyl-5-methoxy- (6CI)
- Spectra-Sorb U.V. 284
- 3-Benzoyl-4-hydroxy-6-methoxybenzenesulfonic acid
- Sungard
- Benzophenone 4
- Uval
- Sulisobenzona
- Sulisobenzonum
- Uvinul
- Seesorb 101S
- Syntase 230
- Uvinul MS 40
- Benzenesulfonic acid, 5-benzoyl-4-hydroxy-2-methoxy-
- Spectra-Sorb UV 284
- Uvinuc ms 40
| Evaluate |

