| "Descrizione" by Nat45 (5724 pt) | 2025-Jan-07 14:30 |
Review Consensus: 15 Rating: 7.5 Number of users: 2
| Evaluation | N. Experts | Evaluation | N. Experts |
|---|---|---|---|
| 1 | 6 | ||
| 2 | 7 | ||
| 3 | 8 | ||
| 4 | 9 | ||
| 5 | 10 |
Sodium Ascorbate, the sodium salt of ascorbic acid (vitamin C), is a water-soluble compound widely used in cosmetics, food, and pharmaceuticals. Known for its antioxidant properties, it protects the skin from oxidative stress, supports collagen synthesis, and brightens the skin tone. In formulations, it acts as a stabilizer and preservative, making it a versatile ingredient in skincare and anti-aging products.
Chemical Composition and Structure
Sodium Ascorbate has the chemical formula C6H7NaO6.
Core Structure:
- Derived from ascorbic acid by neutralizing one of its acidic groups with sodium, enhancing stability and water solubility.
Properties:
- Retains the antioxidant properties of ascorbic acid.
- Exhibits reduced acidity, making it gentler on the skin and stomach compared to pure vitamin C.
Physicochemical Properties
- Appearance: White to pale yellow crystalline powder.
- Odor: Odorless.
- Solubility: Highly soluble in water.
- Stability: More stable than ascorbic acid in aqueous solutions, though sensitive to heat, light, and air.
- pH: Neutral to slightly alkaline in aqueous solutions.
Production Process
Neutralization:
- Ascorbic acid reacts with sodium carbonate or sodium hydroxide to form sodium ascorbate.
Purification:
- The resulting solution is purified through filtration and recrystallization to remove impurities.
Drying and Packaging:
- The purified product is dried and packaged under controlled conditions to preserve its antioxidant properties.
Applications
Medical Applications
Vitamin C Supplementation:
- Used in oral and injectable forms to prevent and treat vitamin C deficiencies.
- Supports immune function and wound healing.
Antioxidant Therapy:
- Reduces oxidative stress in chronic conditions and promotes recovery.
Cosmetics
Antioxidant Protection:
- Protects the skin from free radical damage caused by UV radiation and environmental stressors.
Collagen Synthesis:
- Promotes collagen production, improving skin elasticity and reducing the appearance of fine lines and wrinkles.
Brightening Effect:
- Reduces melanin production, helping to lighten dark spots and even out skin tone.
Stabilizer:
- Used to stabilize other active ingredients in formulations, enhancing product longevity.
Food Applications
- Acts as a preservative in food products, preventing oxidation and maintaining freshness.
- Used as a vitamin C source in fortified foods and beverages.
Industrial Applications
- Used in formulations requiring a stable, water-soluble antioxidant.
- Found in natural and organic product lines due to its multifunctional benefits.
Environmental and Safety Considerations
Biodegradability:
- Sodium Ascorbate is biodegradable and poses no significant environmental risk.
Safety Profile:
- Considered safe for cosmetic and pharmaceutical use within regulated concentrations.
- Non-irritating and suitable for sensitive skin formulations.
Sustainability:
- Derived from naturally occurring ascorbic acid, with low environmental impact during production.
Studies
Food
It is an antioxidant used mostly in the food sector where it is labeled with the number E301 in food additives.
Medical
In dentistry it is used in teeth whitening (1) and as a binder against micro-infiltration (2).
Cosmetics
Antioxidant agent. Ingredient that counteracts oxidative stress and prevents cell damage. Free radicals, pathological inflammatory processes, reactive nitrogen species and reactive oxygen species are responsible for the ageing process and many diseases caused by oxidation.
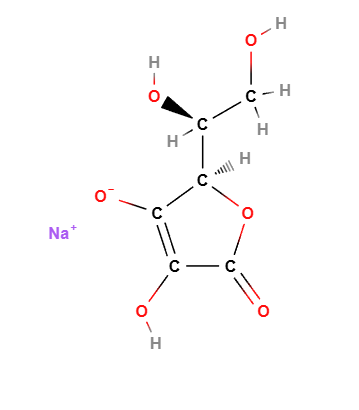 |  |
Molecular Formula : C6H7O6Na C6H7NaO6
Molecular Weight: 198.106 g/mol
CAS: 134-03-2 58657-35-5
EC Number: 205-126-1
UNIII: S033EH8359
Synonyms:
- L-Ascorbic acid sodium salt
- L-Ascorbic acid, monosodium salt
- Vitamin C sodium
- E301
- Monosodium L-ascorbate
- Ascorbicin
- Ascorbic acid sodium salt
- Sodascorbate
- Ascorbin
- Cebitate
- Ascorbic acid sodium derivative
- Aminofenitrooxon
- Sodium derivative of 3-oxo-L-gulofuranolactone
- Natrii ascorbas
- sodium (2R)-2-[(1S)-1,2-dihydroxyethyl]-4-hydroxy-5-oxo-2,5-dihydrofuran-3-olate
- Sodium (R)-2-((S)-1,2-dihydroxyethyl)-4-hydroxy-5-oxo-2,5-dihydrofuran-3-olate
- sodium;(2R)-2-[(1S)-1,2-dihydroxyethyl]-4-hydroxy-5-oxo-2H-furan-3-olate
Bibliografia____________________________________________________________________
(1) Güler E, Gönülol N, Özyilmaz ÖY, Yücel AÇ. Effect of sodium ascorbate on the bond strength of silorane and methacrylate composites after vital bleaching. Braz Oral Res. 2013 Jul-Aug;27(4):299-304. doi: 10.1590/s1806-83242013000400002.
Abstract. We investigated the effect of sodium ascorbate (SA) on the microtensile bond strengths (MTBSs) of different composites to bovine enamel after vital bleaching with hydrogen peroxide (HP) or carbamide peroxide (CP). Thirty bovine incisors were randomly divided into five groups and treated with no bleaching application (control), 35% HP alone, 35% HP+10% SA for 10 minutes (HP+SA), 16% CP alone, or 16% CP+10% SA for 10 minutes (CP+SA). Specimens were restored with Silorane adhesive and Filtek Silorane composite (designated as S/group) or with Clearfil SE bond and Filtek Supreme XT (designated as F/group). Composite build-up was created on the enamel. Sectioned specimens (n=10 per group; 1 mm2; cross-sectional area) were created and stressed in a universal testing machine at 1 mm/min crosshead speed. The application of 10% SA immediately after bleaching with 16% CP or 35% HP increased the enamel MTBS, regardless of the adhesive/composite resin used. The resulting MTBS values were similar to those of the control groups. Use of 16% CP and 35% HP alone decreased the enamel MTBS, regardless of the adhesive/composite resin used, with F/CP+SA=F/HP+SA=F/CP=S/CP+SA=S/HP+SA=S/C>S/CP=S/HP=F/CP=F/HP (p<0.05). We concluded that the application of SA for 10 minutes immediately after vital bleaching increases the enamel BS for dimethacrylate- and silorane-based composites.
(2) Yoon M, Burrow MF, Wong R, Parashos P. Effect of sodium ascorbate on resin bonding to sodium perborate-bleached dentin. Oper Dent. 2014 Jan-Feb;39(1):98-106. doi: 10.2341/12-516-L.
Abstract. SUMMARY This was an in vitro study to evaluate the effect of sodium ascorbate on the microshear bond strength (MSBS) of resin composite to sodium perborate-bleached dentin. Molar dentin sections were divided into six groups: 1) control, 2) sodium perborate (SP) bleach and immediate bonding, 3) SP and 30 second sodium ascorbate (SA); 4) SP and 1 minute SA; 5) SP and 2 minute SA; and 6) SP and 7 day delay before bonding. They were further divided into two-step self-etching (Clearfil SE Bond) or all-in-one self-etching (Xeno IV) adhesive systems. Resin composite microtubes were bonded according to dentin location-center, pulp horn, and peripheral positions-and an MSBS test was carried out. Failure mode was determined using light microscopy and scanning electron microscopy. There were no significant differences between the treatment types/groups. MSBSs were significantly higher for two-step self-etching adhesive compared with all-in-one self-etching adhesive (p=0.028). For the all-in-one adhesive, MSBSs at the center and pulp horn positions were significantly lower than the peripheral positions (p<0.001). All-in-one groups had significantly more adhesive failures than two-step adhesive groups (p=0.015). The odds of adhesive failure were higher at the pulp horn position than the peripheral position (p=0.004). Sodium perborate bleaching of dentin had no effect on MSBS or mode of failure for either two-step or all-in-one self-etching adhesives; therefore, the effect of sodium ascorbate was negligible. The two-step adhesive groups demonstrated the highest MSBS, and the all-in-one groups, when bonded to center and pulp horn dentin, exhibited the lowest MSBS.
Park JY, Kwon TY, Kim YK. Effective application duration of sodium ascorbate antioxidant in reducing microleakage of bonded composite restoration in intracoronally-bleached teeth. Restor Dent Endod. 2013 Feb;38(1):43-7. doi: 10.5395/rde.2013.38.1.43.
Abstract. Objectives: The aim of this study was to determine an appropriate application duration of sodium ascorbate (SA) antioxidant gel in reducing microleakage of bonded composite restoration in intracoronally-bleached teeth....Results: Group IB showed a significantly higher microleakge than the control group (p = 0.006) and group DB a statistically similar score to the control group (p > 0.999). Although groups S10m, S60m, and S24h exhibited significantly higher scores than group DB (p < 0.05), the microleakage in groups S3d and S7d was statistically similar to that in group DB (p = 0.771, p > 0.999). Conclusions: Application of SA gel for 3 day after nonvital bleaching was effective in reducing microleakage of composite restoration in intracoronally-bleached teeth.
| Evaluate |

