| "Descrizione" by Nat45 (5724 pt) | 2024-Jun-21 10:49 |
Review Consensus: 10 Rating: 10 Number of users: 1
| Evaluation | N. Experts | Evaluation | N. Experts |
|---|---|---|---|
| 1 | 6 | ||
| 2 | 7 | ||
| 3 | 8 | ||
| 4 | 9 | ||
| 5 | 10 |
Hyaluronic acid (HA) is a polysaccharide belonging to the class of glycosaminoglycans, involved in tissue repair processes and in their regeneration. It is a fundamental component of the extracellular matrix, which contributes to an optimal performance of the repair processes by providing the appropriate degree of hydration, which facilitates cell migration (1).
Hyaluronic acid is a natural component present in abundance in load-bearing joints of the human body (2), has the property of retaining skin moisture (3), has anti-inflammatory, antioxidant, and antibacterial effects for the treatment of periodontal diseases ( 4). However, the lubricating effect of hyaluronic acid is generally of short duration and the duration of its biological effects is not predictable (5).
Hyaluronic Acid is a naturally occurring substance in the human body, widely used in various cosmetic and personal care products for its exceptional moisturizing and anti-aging properties. It provides long-lasting hydration, enhances skin elasticity, and improves overall skin texture.
Chemical Composition and Structure
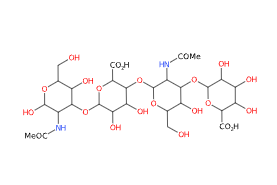
Hyaluronic Acid, chemically known as a glycosaminoglycan, consists of repeating disaccharide units of N-acetylglucosamine and glucuronic acid. Its unique ability to bind and retain water molecules contributes to its remarkable moisturizing properties. The molecular formula for Hyaluronic Acid is (C14H21NO11)n, where "n" represents the number of repeating units.
Physical Properties
Hyaluronic Acid typically appears as a white, odorless powder that is highly soluble in water. When dissolved, it forms a clear, viscous solution that can hold up to 1,000 times its weight in water, making it an excellent hydrating agent. Its viscosity and water-retaining capacity make it a popular ingredient in various cosmetic formulations.
Industrial Production Process
- Preparation of reagents. The main raw materials include natural sources (such as rooster combs) or genetically modified bacteria (Escherichia coli or Streptococcus zooepidemicus) for fermentation.
- Fermentation. If using a fermentation method, the bacteria are cultured in a nutrient-rich growth medium, such as glucose, peptones, and mineral salts, at controlled temperature and pH. The fermentation is maintained for a specified period to produce hyaluronic acid.
- Harvesting. At the end of fermentation, the fermentation broth is centrifuged to separate the bacterial cells from the supernatant containing hyaluronic acid.
- Purification. The supernatant undergoes several purification steps, including filtration, solvent precipitation with ethanol, and chromatography to remove impurities and concentrate the hyaluronic acid.
- Precipitation. The hyaluronic acid is precipitated by adding ethanol or another organic solvent. The precipitate is collected by centrifugation or filtration.
- Washing. The hyaluronic acid precipitate is washed with ethanol or water to remove further impurities.
- Drying. The washed precipitate is dried by lyophilization (freeze-drying) or vacuum drying to obtain a dry product.
- Grinding. The dried product is ground to obtain a fine and uniform powder.
- Classification. The dried powder is classified to ensure a uniform particle size. This step may involve sieving or the use of air classifiers.
- Stabilization. The hyaluronic acid is stabilized to ensure its stability during transportation and storage, preventing aggregation and degradation.
- Quality control. The hyaluronic acid undergoes rigorous quality testing to ensure it meets standards for purity, safety, and functionality. These tests include chemical analysis, spectroscopy, and physical tests to determine particle size and rheological properties.
Industrially it is in the form of a white, water-soluble powder.

Applications
Moisturizers and Creams: Hyaluronic Acid is widely used in moisturizers and creams for its ability to provide intense hydration. It helps maintain the skin's moisture balance, resulting in smoother and more supple skin.
Anti-Aging Products: Due to its strong water-binding capacity, Hyaluronic Acid is a key ingredient in anti-aging products. It helps to plump the skin, reducing the appearance of fine lines and wrinkles, and enhancing skin firmness and elasticity.
Serums and Essences: In serums and essences, Hyaluronic Acid acts as a powerful hydrating agent, improving the penetration and effectiveness of other active ingredients. It provides immediate and long-lasting hydration, making the skin appear more radiant and youthful.
Hair Care Products: Hyaluronic Acid is also used in hair care products such as shampoos, conditioners, and hair masks. It helps to hydrate and condition the hair, improving its texture, softness, and manageability.
Eye Care: The ingredient is ideal for use in eye creams and gels due to its gentle yet effective moisturizing properties. It helps to reduce puffiness, fine lines, and dark circles around the delicate eye area.

Safety
Hyaluronic Acid is generally considered safe for use in cosmetic and personal care products. It is non-irritating, non-sensitizing, and suitable for all skin types, including sensitive skin. As a biodegradable substance, it poses minimal risk to the environment when disposed of properly. However, it is important to use Hyaluronic Acid within the recommended guidelines to ensure safety and efficacy.
For more information:
| Appearance | White powder |
| Boiling Point | 1274.4±65.0°C at 760 mmHg |
| Flash Point | 724.5±34.3°C |
| Density | 1.8±0.1 g/cm3 |
| PSA | 399.71000 |
| LogP | -6.62 |
| Refraction Index | 1.666 |
| Vapor Pressure | 0.0±0.6 mmHg at 25°C |
| Storage | −20°C |
 |  |
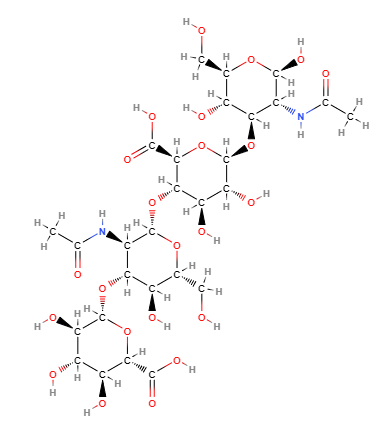 |  |
FMolecular Formula C28H44N2O23
Molecular Weight 776.6
CAS 9004-61-9
UNII
EC Number
DSSTox Substance ID
IUPAC (2S,3S,4S,5R,6R)-6-[(2S,3R,5S,6R)-3-acetamido-2-[(2S,3S,4R,5R,6R)-6-[(2R,3R,5S,6R)-3-acetamido-2,5-dihydroxy-6-(hydroxymethyl)oxan-4-yl]oxy-2-carboxy-4,5-dihydroxyoxan-3-yl]oxy-5-hydroxy-6-(hydroxymethyl)oxan-4-yl]oxy-3,4,5-trihydroxyoxane-2-carboxylic acid
InChI=1S/C28H44N2O23/c1-5(33)29-9-18(11(35)7(3-31)47-25(9)46)49-28-17(41)15(39)20(22(53-28)24(44)45)51-26-10(30-6(2)34)19(12(36)8(4-32)48-26)50-27-16(40)13(37)14(38)21(52-27)23(42)43/h7-22,25-28,31-32,35-41,46H,3-4H2,1-2H3,(H,29,33)(H,30,34)(H,42,43)(H,44,45)/t7-,8-,9-,10-,11-,12-,13+,14+,15-,16-,17-,18?,19?,20+,21+,22+,25-,26+,27-,28-/m1/s1
InChl Key KIUKXJAPPMFGSW-MNSSHETKSA-N
SMILES CC(=O)NC1C(C(C(OC1O)CO)O)OC2C(C(C(C(O2)C(=O)O)OC3C(C(C(C(O3)CO)O)OC4C(C(C(C(O4)C(=O)O)O)O)O)NC(=O)C)O)O
Synonyms
- (2S,3S,4S,5R,6R)-6-[(2S,3R,5S,6R)-3-acetamido-2-[(2S,3S,4R,5R,6R)-6-[(2R,3R,5S,6R)-3-acetamido-2,5-dihydroxy-6-(hydroxymethyl)oxan-4-yl]oxy-2-carboxy-4,5-dihydroxyoxan-3-yl]oxy-5-hydroxy-6-(hydroxymethyl)oxan-4-yl]oxy-3,4,5-trihydroxyoxane-2-carboxylic acid
- (2S,3S,4S,5R,6R)-6-[(2S,3R,5S,6R)-3-acetamido-2-[(2S,3S,4R,5R,6R)-6-[(2R,3R,5S,6R)-3-acetamido-2,5-dihydroxy-6-(hydroxymethyl)oxan-4-yl]oxy-2-carboxy-4,5-dihydroxyoxan-3-yl]oxy-5-hydroxy-6-(hydroxymethyl)oxan-4-yl]oxy-3,4,5-trihydroxyoxane-2-carboxylic acid
- D-Glucopyranose, O(4ξ)-α-D-xylo-hexopyranuronosyl-(1->3)-O-2-(acetylamino)-2-deoxy-β-D-allopyranosyl-(1->4)-O(5ξ)-β-D-xylo-hexopyranuronosyl-(1->3)-2-(acetylamino)-2-deoxy-
References_________________________________________________________________________________
(1) Laliscia C, Delishaj D, Fabrini MG, Gonnelli A, Morganti R, Perrone F, Tana R, Paiar F, Gadducci A. Acute and late vaginal toxicity after adjuvant high-dose-rate vaginal brachytherapy in patients with intermediate risk endometrial cancer: is local therapy with hyaluronic acid of clinical benefit? J Contemp Brachytherapy. 2016 Dec;8(6):512-517. doi: 10.5114/jcb.2016.64511.
Abstract. Purpose: The aim of the present study was to evaluate the effectiveness of hyaluronic acid (HA) in the prevention of acute and late vaginal toxicities after high-dose-rate (HDR) vaginal brachytherapy (BT).....Conclusions: These results appear to suggest that the local therapy with HA is of clinical benefit for intermediate risk endometrial cancer patients who receive adjuvant HDR-vaginal BT after surgery. A randomized trial comparing HA treatment vs. no local treatment in this clinical setting is warranted to further evaluate the efficacy of HA in preventing vaginal BT-related vaginal toxicity.
(2) Correia CR, Moreira-Teixeira LS, Moroni L, Reis RL, van Blitterswijk CA, Karperien M, Mano JF. Chitosan scaffolds containing hyaluronic acid for cartilage tissue engineering. Tissue Eng Part C Methods. 2011 Jul;17(7):717-30. doi: 10.1089/ten.tec.2010.0467.
Abstract. Scaffolds derived from natural polysaccharides are very promising in tissue engineering applications and regenerative medicine, as they resemble glycosaminoglycans in the extracellular matrix (ECM). In this study, we have prepared freeze-dried composite scaffolds of chitosan (CHT) and hyaluronic acid (HA) in different weight ratios containing either no HA (control) or 1%, 5%, or 10% of HA. We hypothesized that HA could enhance structural and biological properties of CHT scaffolds. To test this hypothesis, physicochemical and biological properties of CHT/HA scaffolds were evaluated. Scanning electron microscopy micrographs, mechanical properties, swelling tests, enzymatic degradation, and Fourier transform infrared (FTIR) chemical maps were performed. To test the ability of the CHT/HA scaffolds to support chondrocyte adhesion and proliferation, live-dead and MTT assays were performed. Results showed that CHT/HA composite scaffolds are noncytotoxic and promote cell adhesion. ECM formation was further evaluated with safranin-O and alcian blue staining methods, and glycosaminoglycan and DNA quantifications were performed. The incorporation of HA enhanced cartilage ECM production. CHT/5HA had a better pore network configuration and exhibited enhanced ECM cartilage formation. On the basis of our results, we believe that CHT/HA composite matrixes have potential use in cartilage repair.
(3) Kablik J, Monheit GD, Yu L, Chang G, Gershkovich J. Comparative physical properties of hyaluronic acid dermal fillers. Dermatol Surg. 2009 Feb;35 Suppl 1:302-12. doi: 10.1111/j.1524-4725.2008.01046.x.
Abstract. Background: Hyaluronic acid (HA) fillers are becoming the material of choice for use in cosmetic soft tissue and dermal correction. HA fillers appear to be similar, but their physical characteristics can be quite different. These differences have the potential to affect the ability of the physician to provide the patient with a natural and enduring result.....Conclusion: Combining the objective factors that influence filler performance with clinical experience will provide the patient with the optimal product for achieving the best cosmetic result. A careful review of these gel characteristics is essential in determining filler selection, performance, and patient expectations.
(4) Jentsch H, Pomowski R, Kundt G, Göcke R. Treatment of gingivitis with hyaluronan. J Clin Periodontol. 2003 Feb;30(2):159-64. doi: 10.1034/j.1600-051x.2003.300203.x.
Abstract. Objectives: Hyaluronic acid (hyaluronan) is a glycosaminoglycan with anti-inflammatory and antiedematous properties. It was evaluated in a gel formulation for its effect in the treatment of plaque-induced gingivitis.....Conclusions: These data suggest that a hyaluronan containing gel has a beneficial effect in the treatment of plaque-induced gingivitis.
(5) Huang YC, Huang KY, Yang BY, Ko CH, Huang HM. Fabrication of Novel Hydrogel with Berberine-Enriched Carboxymethylcellulose and Hyaluronic Acid as an Anti-Inflammatory Barrier Membrane. Biomed Res Int. 2016;2016:3640182. doi: 10.1155/2016/3640182.
Abstract. An antiadhesion barrier membrane is an important biomaterial for protecting tissue from postsurgical complications. However, there is room to improve these membranes. Recently, carboxymethylcellulose (CMC) incorporated with hyaluronic acid (HA) as an antiadhesion barrier membrane and drug delivery system has been reported to provide excellent tissue regeneration and biocompatibility. The aim of this study was to fabricate a novel hydrogel membrane composed of berberine-enriched CMC prepared from bark of the P. amurense tree and HA (PE-CMC/HA). In vitro anti-inflammatory properties were evaluated to determine possible clinical applications. The PE-CMC/HA membranes were fabricated by mixing PE-CMC and HA as a base with the addition of polyvinyl alcohol to form a film. Tensile strength and ultramorphology of the membrane were evaluated using a universal testing machine and scanning electron microscope, respectively. Berberine content of the membrane was confirmed using a UV-Vis spectrophotometer at a wavelength of 260 nm. Anti-inflammatory property of the membrane was measured using a Griess reaction assay. Our results showed that fabricated PE-CMC/HA releases berberine at a concentration of 660 μg/ml while optimal plasticity was obtained at a 30 : 70 PE-CMC/HA ratio. The berberine-enriched PE-CMC/HA had an inhibited 60% of inflammation stimulated by LPS. These results suggest that the PE-CMC/HA membrane fabricated in this study is a useful anti-inflammatory berberine release system.
| Evaluate |

