| "Descrizione" by Frank123 (12058 pt) | 2023-Jul-28 16:31 |
Review Consensus: 9 Rating: 9 Number of users: 1
| Evaluation | N. Experts | Evaluation | N. Experts |
|---|---|---|---|
| 1 | 6 | ||
| 2 | 7 | ||
| 3 | 8 | ||
| 4 | 9 | ||
| 5 | 10 |
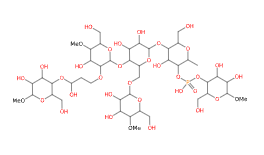
E1442 (Hydroxy propyl distarch phosphate) is a modified starch obtained by esterification of a food starch (rice, maize) phosphorus oxychloride or sodium trimetaphosphate and propylene oxide.
Breakdown of the name and function of the components
- Hydroxy Propyl - Indicates that starch molecules have been modified with hydroxypropyl groups.
- Distarch - Refers to a modified starch.
- Phosphate - Indicates the presence of phosphate groups as part of the modification.
Description and function of the raw materials used in production
- Starch - A polysaccharide extracted mainly from cereals and tubers.
- Propylene oxide - Used to introduce hydroxypropyl groups into starch.
- Phosphate - Used to introduce phosphate groups into starch.
Summary of its industrial synthesis process step by step
- Starch extraction - Starch is extracted from plants like corn, potatoes, or wheat.
- Hydroxypropylation - The extracted starch is treated with propylene oxide to introduce hydroxypropyl groups.
- Phosphation - The hydroxypropylated starch is then treated with a phosphating agent to introduce phosphate groups.
- Purification - The modified starch is purified to remove any unreacted reagents or impurities.
- Drying - The product is dried to remove residual moisture.
It appears as a white powder or white granules.

What it is used for and where
Food
Ingredient included in the list of European food additives as E1442 with a thickening and stabilising function. It is used in ketchup as it showed lower values of the K parameter.
Applications
- Food Additive - Acts as a thickener, stabilizer, and emulsifier in various food products.
- Texture Improvement - Used in foods like sauces, soups, and ice creams to improve consistency and stability.
- Resistance to Harsh Conditions - Provides enhanced stability to food preparations undergoing processes such as pasteurization, sterilization, or freezing.
- Baked Goods - Can be used in baked goods to enhance softness and shelf life.
Medical
A few studies have looked at the effects of hydroxypropyl distarch phosphate on postprandial energy metabolism, and the findings suggest that this modified starch may have beneficial implications in weight management (1).
Safety
The EFSA Panel on Food Additives and Nutrient Sources Added to Food considers that there is no safety concern for the use of modified starches as food additives at the uses and use levels declared for the general population and that a numerical ADI is not necessary (2).
 |  |
 |  |
- Molecular Formula C44H79O35P
- Molecular Weight 1199.0
- CAS 53124-00-8
- UNII
- EC Number 610-966-0
References_____________________________________________________________________
(1) Shimotoyodome, A., Suzuki, J., Kameo, Y. and Hase, T., 2011. Dietary supplementation with hydroxypropyl-distarch phosphate from waxy maize starch increases resting energy expenditure by lowering the postprandial glucose-dependent insulinotropic polypeptide response in human subjects. British journal of nutrition, 106(1), pp.96-104.
Abstract. The aim of the present study was to investigate the effects of hydroxypropyl-distarch phosphate (HDP) supplementation on postprandial energy metabolism and glucose-dependent insulinotropic polypeptide (GIP) in human subjects. A total of ten healthy male subjects, with a mean BMI of 23·6 (SEM 1·3) kg/m2, age 35·2 (SEM 1·9) years and body weight 71·1 (SEM 4·0) kg, participated in a randomised, cross-over, intervention study with two different test meals (1673·6 kJ) containing either waxy maize starch or HDP from waxy maize starch (degree of substitution 0·154, P content 0·004 %). Resting energy expenditure (REE) and blood concentrations of various biomarkers were measured at fasting and up to 180 min postprandially. Indirect calorimetry showed that the HDP meal caused higher REE (P < 0·05) and fat utilisation (P < 0·001) than the waxy maize starch meal. The HDP meal led to significantly lower postprandial glucose (P < 0·05), insulin (P < 0·05) and GIP (P < 0·05) responses than the waxy maize starch meal. Both postprandial REE (R − 0·576, P < 0·01) and fat utilisation (R − 0·514, P < 0·05) were negatively correlated with the postprandial GIP response, but not with the glucose and insulin responses. In conclusion, dietary supplementation with HDP lowers postprandial GIP and increases postprandial REE and fat utilisation in healthy humans. An HDP-rich diet may therefore have beneficial implications in weight management. Further studies are required to confirm the efficacy in overweight or obese subjects, and to determine the precise mechanisms.
(2) EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS), Mortensen, A., Aguilar, F., Crebelli, R., Di Domenico, A., Dusemund, B., Frutos, M.J., Galtier, P., Gott, D., Gundert‐Remy, U. and Lambré, C., 2017. Re‐evaluation of oxidised starch (E 1404), monostarch phosphate (E 1410), distarch phosphate (E 1412), phosphated distarch phosphate (E 1413), acetylated distarch phosphate (E 1414), acetylated starch (E 1420), acetylated distarch adipate (E 1422), hydroxypropyl starch (E 1440), hydroxypropyl distarch phosphate (E 1442), starch sodium octenyl succinate (E 1450), acetylated oxidised starch (E 1451) and starch aluminium octenyl succinate (E 1452) as food additives. EFSA Journal, 15(10), p.e04911.
Abstract. Following a request from the European Commission, the EFSA Panel on Food Additives and Nutrient sources added to Food (ANS) was asked to deliver a scientific opinion on the re-evaluation of 12 modified starches (E 1404, E 1410, E 1412, E 1413, E 1414, E 1420, E 1422, E 1440, E 1442, E 1450, E 1451 and E 1452) authorised as food additives in the EU in accordance with Regulation (EC) No 1333/2008 and previously evaluated by JECFA and the SCF. Both committees allocated an acceptable daily intake (ADI) ‘not specified’. In humans, modified starches are not absorbed intact but significantly hydrolysed by intestinal enzymes and then fermented by the intestinal microbiota. Using the read-across approach, the Panel considered that adequate data on short- and long-term toxicity and carcinogenicity, and reproductive toxicity are available. Based on in silico analyses, modified starches are considered not to be of genotoxic concern. No treatment-related effects relevant for human risk assessment were observed in rats fed very high levels of modified starches (up to 31,000 mg/kg body weight (bw) per day). Modified starches (e.g. E 1450) were well tolerated in humans up to a single dose of 25,000 mg/person. Following the conceptual framework for the risk assessment of certain food additives, the Panel concluded that there is no safety concern for the use of modified starches as food additives at the reported uses and use levels for the general population and that there is no need for a numerical ADI. The combined exposure to E 1404–E 1451 at the 95th percentile of the refined (brand-loyal) exposure assessment scenario for the general population was up to 3,053 mg/kg bw per day. Exposure to E 1452 for food supplement consumers only at the 95th percentile was up to 22.1 mg/kg bw per day.
| Evaluate |

