| "Descrizione" by FRanier (9971 pt) | 2023-Nov-17 11:23 |
Review Consensus: 9 Rating: 9 Number of users: 1
| Evaluation | N. Experts | Evaluation | N. Experts |
|---|---|---|---|
| 1 | 6 | ||
| 2 | 7 | ||
| 3 | 8 | ||
| 4 | 9 | ||
| 5 | 10 |
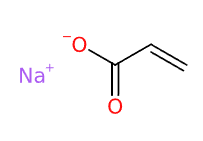
Sodium acrylate (2- propenoic acid, homopolymer, sodium salt, sulfonated) is a water-soluble, high molecular weight compound with high rigidity and viscosity in its aqueous solution.
It is the sodium salt of acrylic acid. It's often used as a monomer in the production of superabsorbent polymers, like sodium polyacrylate, which are employed in applications such as diapers and sanitary napkins.
The name describes the structure of the molecule -
- Sodium - Indicates the presence of sodium ions.
- Acrylate - Indicates that the molecule is a salt derived from acrylic acid.
Description of raw materials used in production -
- Acrylic acid - It's the acidic form that serves as the starting point to produce the sodium acrylate salt.
- Sodium hydroxide or another sodium salt - Used to neutralize the acrylic acid and form sodium acrylate.
Synthesis process -
- Neutralization - Acrylic acid is neutralized with a sodium base, such as sodium hydroxide, to form sodium acrylate.
It appears in the form of a white powder.

What it is for and where
Cosmetics
Absorbent. Absorbs substances dispersed or dissolved in aqueous solutions, water/oil, oil/water.
Binder agent. Ingredient that is used in cosmetic, food and pharmaceutical products as an anti-caking agent with the function of making the product in which it is incorporated silky, compact and homogenous. The binder, either natural such as mucilage, gums and starches or chemical, may be in the form of a powder or liquid.
Skin conditioning agent - Emollient. Emollients have the characteristic of enhancing the skin barrier through a source of exogenous lipids that adhere to the skin, improving barrier properties by filling gaps in intercorneocyte clusters to improve hydration while protecting against inflammation. In practice, they have the ability to create a barrier that prevents transepidermal water loss. Emollients are described as degreasing or refreshing additives that improve the lipid content of the upper layers of the skin by preventing degreasing and drying of the skin. The problem with emollients is that many have a strong lipophilic character and are identified as occlusive ingredients; they are oily and fatty materials that remain on the skin surface and reduce transepidermal water loss. In cosmetics, emollients and moisturisers are often considered synonymous with humectants and occlusives.
Emulsion stabiliser. Emulsions are thermodynamically unstable. Emulsion stabilisers improve the formation and stability of single and double emulsions. as well as their shelf-life. It should be noted that in the structure-function relationship, the molar mass of the ingredient used plays an important role.
Film-forming agent. It produces, upon application, a very thin continuous film with an optimal balance of cohesion, adhesion and stickiness on skin, hair or nails to counteract or limit damage from external phenomena such as chemicals, UV rays and pollution.
Hair fixative. This ingredient has the ability to create, with its protective film, stiffness and hold in the hair, and also has the ability to form, with its hydrophilic and elastic properties, bonds between the hair fibres, to keep the hair in a particular shape for a certain time. In short, it allows physical control of the hairstyle.
Skin conditioning agent. It is the mainstay of topical skin treatment as it has the function of restoring, increasing or improving skin tolerance to external factors, including melanocyte tolerance. The most important function of the conditioning agent is to prevent skin dehydration, but the subject is rather complex and involves emollients and humectants that can be added in the formulation.
Viscosity control agent. It controls and adapts, Increasing or decreasing, viscosity to the required level for optimal chemical and physical stability of the product and dosage in gels, suspensions, emulsions, solutions.
Applications
Superabsorbent Materials - Used in baby diapers, incontinence products, and feminine hygiene products due to its ability to absorb large amounts of liquid.
Water Treatment - Acts as a flocculating agent, helping to remove impurities from water.
Agriculture - Used to enhance water retention in soils, particularly in arid areas or under drought conditions.
Controlled Release - Can be utilized in controlled release systems for drugs or other substances.
Medicine
Studies
- In the present study, preventive effects of Sodium polyacrylate (PANa) on three kinds of esophageal lesions induced by gastric juice were examined in comparison with those of aceglutamide aluminium and sodium alginate. The influences of PANa on gastric contents were also studied. The preventive effect of PANa given intraesophageally on esophageal lesions induced by the intraesophageal application of gastric juice was more potent than aceglutamide aluminium and sodium alginate. Oral administration of PANa inhibited the formation of esophageal ulcer by pylorus ligation more markedly than aceglutamide aluminium, whereas sodium alginate had no effect in a high dose of 500 mg/kg. In preventing gastric ulcer which occurred simultaneously with the esophageal ulcer after the pylorus ligation, aceglutamide aluminium was most potent, and PANa was as potent as sodium alginate. Oral administration of PANa showed a more protective effect than aceglutamide aluminium on the esophageal ulceration induced by the simultaneous ligations of the pylorus and limiting ridge, whereas sodium alginate in a high dose of 500 mg/kg had little effect on the ulcer formation. PANa caused only a slight increase in the pH of gastric juice and a slight decrease in pepsin activity. From these results, it may be concluded that PANa showed an antiulcerogenic activity mainly due to its mucosa covering action against gastric juice (1).
- Available methods for postoperative adhesion prevention are insufficient. A previous study demonstrated that LM-200, a bioadhesive cellulose derivative was effective in reducing adhesions. Increasing the viscosity of a polymer solution enhances the tissue separating properties. Theoretically, a combination of sodium polyacrylate (PA) and LM-200 would give more viscous solutions than LM-200 alone, and thus be more efficacious. Therefore the efficacy of various combinations of LM-200 and PA was investigated. A lesion was created in the peritoneum of mice. The solutions to be tested, or saline, were given intraperitoneally. One week post-operatively, adhesion formation was quantified and expressed as a percentage of the original lesion covered with adhesions. PA (0.01 and 0.03 wt%) given separately did not differ in adhesion reducing effect from LM-200 (p = 0.3710 and 0.3481) but PA (0.1 wt%) resulted in significantly less adhesion formation (p = 0.0004). The effect of LM-200 increased significantly when adding PA (0.01 wt%) (p = 0.0007) or PA (0.03 wt%) (p < 0.0001). When adding PA (0.1 wt%) the effect was even more pronounced (p < 0.0001). The combination of a bioadhesive cellulose derivative and the polymer PA, was effective in reducing postoperative adhesion formation and a dose-dependent increase in efficacy was obtained compared to using the two components separately (2).
Industry
Self-healing solid-state aqueous rechargeable NiCo||Zn batteries are inherently safe and have a high energy density and mechanical robustness. However, the self-healability of solid-state batteries has only been realized by a few studies in which electron/ion-inactive self-healable substrates are utilized. This arises from the lack of self-healable electrolytes. Now an intrinsically self-healing battery has been designed that utilizes a new electrolyte that is intrinsically self-healable. Sodium polyacrylate hydrogel chains are crosslinked by ferric ions to promote dynamic reconstruction of an integral network. These non-covalent crosslinkers can form ionic bonds to reconnect damaged surfaces when the hydrogel is cut off, providing an ultimate solution to the intrinsic self-healability problem of batteries. As a result, this NiCo||Zn battery with this hydrogel electrolyte can be autonomically self-healed with over 87 % of capacity retained after 4 cycles of breaking/healing. (3).
Other uses
In detergents, which has the function of thickener, it has the characteristic of binding to magnesium and calcium, favoring the action of surfactants.
Typical optimal commercial product characteristics Sodium acrilate liquid
| Appearance | Colourless, viscous liquid. |
| Boiling Point | 141ºC at 760 mmHg |
| Melting Point | >300 °C |
| Flash Point | Flash Point |
| PSA | 40.13000 |
| pH | 6.0-8.0 |
| Purity | 18±0.5 |
| Density (20℃)g/cm3 | 1.150 |
| Free monomer (CH2=CH-COOH) | % ≤0.5 |
| Safety |  |
 |  |
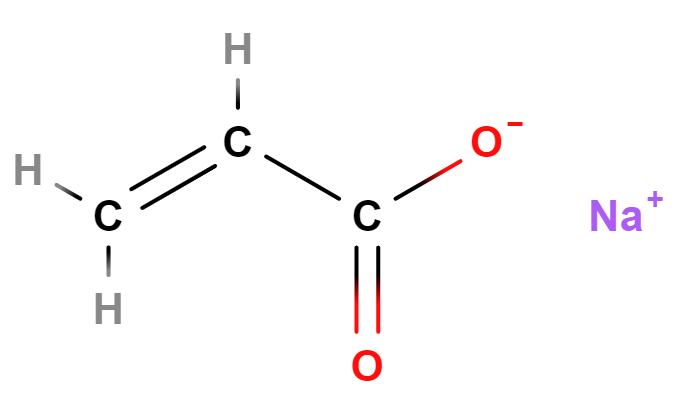 |  |
- Molecular Formula C3H3NaO2 (C3H3O2)n·Na C3H3NaO2)n/CH2=CH-COOH and CH2=CH-COONa
- Molecular Weight 94.045 g/mol
- Exact Mass 94.003075
- CAS: 7446-81-3 9003-04-7 25549-84-2 28603-11-4 95077-68-2
- DSSTox Substance ID DTXSID4027652 DTXSID0049783
- IUPAC sodium;prop-2-enoate
- InChI=1S/C3H4O2.Na/c1-2-3(4)5;/h2H,1H2,(H,4,5);/q;+1/p-1
- InChl Key NNMHYFLPFNGQFZ-UHFFFAOYSA-M
- SMILES C=CC(=O)[O-].[Na+]
- EC Number: 231-209-7 618-349-8 619-104-8 925-839-8
- UNII: 7C98FKB43H
- PubChem Substance ID 24868314
- MDL number MFCD00147949
- ISC 1429
Synonyms :
- 2-Propenoic acid, sodium salt
- Acrylic acid polymer, sodium salt
- Acrylic acid, sodium salt
- carbomer
- carbopol
- HSDB 6087
- LS-145 553
- Polyco
- Sodium polyacrylate
- Sodium poly acrylate
- Polyacrylate sodium salt
- Polyacrylic acid, sodium salt
References______________________________________________________________________
(1) Nakamura K, Ozawa Y, Furuta Y, Miyazaki H. Effects of sodium polyacrylate (PANa) on acute esophagitis by gastric juice in rats. Jpn J Pharmacol. 1982 Jun;32(3):445-56. doi: 10.1254/jjp.32.445. PMID: 7109349.
Abstract. Sodium polyacrylate (PANa) is a water-soluble, high-molecular compound, and its aqueous solution shows a very high viscosity and stringiness. In the present study, preventive effects of PANa on three kinds of esophageal lesions induced by gastric juice were examined in comparison with those of aceglutamide aluminum and sodium alginate. The influences of PANa on gastric contents were also studied. The preventive effect of PANa given intraesophageally on esophageal lesions induced by the intraesophageal application of gastric juice was more potent than aceglutamide aluminum and sodium alginate. Oral administration of PANa inhibited the formation of esophageal ulcer by pylorus ligation more markedly than aceglutamide aluminum, whereas sodium alginate had no effect in a high dose of 500 mg/kg. In preventing gastric ulcer which occurred simultaneously with the esophageal ulcer after the pylorus ligation, aceglutamide aluminum was most potent, and PANa was as potent as sodium alginate. Oral administration of PANa showed a more protective effect than aceglutamide aluminum on the esophageal ulceration induced by the simultaneous ligations of the pylorus and limiting ridge, whereas sodium alginate in a high dose of 500 mg/kg had little effect on the ulcer formation. PANa caused only a slight increase in the pH of gastric juice and a slight decrease in pepsin activity. From the results, it may be concluded that PANa showed an antiulcerogenic activity mainly due to its mucosa covering action against gastric juice.
(2) Falk K, Lindman B, Bengmark S, Larsson K, Holmdahl L. Sodium polyacrylate potentiates the anti-adhesion effect of a cellulose-derived polymer. Biomaterials. 2001 Aug;22(16):2185-90. doi: 10.1016/s0142-9612(00)00360-4.
Abstract. Available methods for postoperative adhesion prevention are insufficient. A previous study demonstrated that LM-200, a bioadhesive cellulose derivative was effective in reducing adhesions. Increasing the viscosity of a polymer solution enhances the tissue separating properties. Theoretically, a combination of sodium polyacrylate (PA) and LM-200 would give more viscous solutions than LM-200 alone, and thus be more efficacious. Therefore the efficacy of various combinations of LM-200 and PA was investigated. A lesion was created in the peritoneum of mice. The solutions to be tested, or saline, were given intraperitoneally. One week post-operatively, adhesion formation was quantified and expressed as a percentage of the original lesion covered with adhesions. PA (0.01 and 0.03 wt%) given separately did not differ in adhesion reducing effect from LM-200 (p = 0.3710 and 0.3481) but PA (0.1 wt%) resulted in significantly less adhesion formation (p = 0.0004). The effect of LM-200 increased significantly when adding PA (0.01 wt%) (p = 0.0007) or PA (0.03 wt%) (p < 0.0001). When adding PA (0.1 wt%) the effect was even more pronounced (p < 0.0001). The combination of a bioadhesive cellulose derivative and the polymer PA, was effective in reducing postoperative adhesion formation and a dose-dependent increase in efficacy was obtained compared to using the two components separately.
(3) Huang Y, Liu J, Wang J, Hu M, Mo F, Liang G, Zhi C. An Intrinsically Self-Healing NiCo||Zn Rechargeable Battery with a Self-Healable Ferric-Ion-Crosslinking Sodium Polyacrylate Hydrogel Electrolyte. Angew Chem Int Ed Engl. 2018 Jul 26;57(31):9810-9813. doi: 10.1002/anie.201805618. Epub 2018 Jul 5
| Evaluate |

