Sodium laurate
Review Consensus: 10 Rating: 10 Number of users: 1
| "Descrizione" by Frank123 (12058 pt) | 2023-Jul-19 10:29 |
Review Consensus: 10 Rating: 10 Number of users: 1
| Evaluation | N. Experts | Evaluation | N. Experts |
|---|---|---|---|
| 1 | 6 | ||
| 2 | 7 | ||
| 3 | 8 | ||
| 4 | 9 | ||
| 5 | 10 |
Sodium laurate is a chemical compound, the sodium salt of lauric acid.
Description of the raw materials:
- Coconut oil or palm oil. Coconut oil or palm oil is one of the main raw materials for the production of sodium laurate. These vegetable oils contain fatty acids, including lauric acid.
- Caustic soda. Caustic soda (sodium hydroxide) is used to convert the lauric acid present in coconut oil or palm oil into sodium laurate. Caustic soda reacts with lauric acid to form the corresponding sodium salt.
Summary of the industrial chemical synthesis process step-by-step:
- Preparation of ingredients. Coconut oil or palm oil is treated to separate the fatty acids, including lauric acid. Caustic soda is prepared in solution.
- Saponification. Coconut oil or palm oil, along with the caustic soda solution, is mixed and heated in a reactor. During the saponification process, lauric acid reacts with caustic soda to form sodium laurate.
- Cooling and solidification. The reactive mixture is cooled, allowing sodium laurate to solidify. The resulting solid solution is then fragmented into flakes or granules.
- Washing and purification. The sodium laurate flakes or granules are washed to remove any impurities or traces of residual reagents.
- Drying. Sodium laurate undergoes a drying process to remove residual moisture and obtain the final product with the desired purity grade.
- Packaging of sodium laurate into appropriate containers for distribution and use.
It appears as a fine white powder. Stable. Combustible. Incompatible with strong oxidising agents, strong bases, strong acids. Soluble in hot water and hot ethyl alcohol; slightly soluble in cold ethyl alcohol, ether and other organic solvents.

What it is used for and where
Surfactant, an indispensable component in soap
Cosmetics
- Surfactant - Cleansing agent. Cosmetic products used to cleanse the skin utilise the surface-active action that produces a lowering of the surface tension of the stratum corneum, facilitating the removal of dirt and impurities.
- Cleansing agent. Ingredient that cleanses skin without exploiting the surface-active properties that produce a lowering of the surface tension of the stratum corneum.
- Surfactant - Emulsifying agent. Emulsions are thermodynamically unstable and are used to soothe or soften the skin and emulsify, so they need a specific, stabilising ingredient. This ingredient forms a film, lowers the surface tension and makes two immiscible liquids miscible. A very important factor affecting the stability of the emulsion is the amount of the emulsifying agent. Emulsifiers have the property of reducing the oil/water or water/oil interfacial tension, improving the stability of the emulsion and also directly influencing the stability, sensory properties and surface tension of sunscreens by modulating the filmometric performance.
Medical
Reagent
Other uses
Raw material in the textile industry
Typical commercial product characteristics Sodium laurate
| Appearance | White powder |
| Boiling Point | 296.1ºC at 760 mmHg |
| Melting Point | 43.8ºC |
| Flash Point | 134.1ºC |
| Density | 1.102 g/cm3 |
| Loss on drying | ≤4.0% |
| Acidity (H+) | ≤5.0mmol/100g |
| Residue on ignition | 29.0~32.0% |
| Iodine | ≤1.0 |
| Particle size through 200mesh | ≥99.0% |
| Heavy metals | Heavy metals |
| Lead | ≤0.0010% |
| PSA | 40.13000 |
| LogP | 2.65720 |
| Safety |  |
 | |
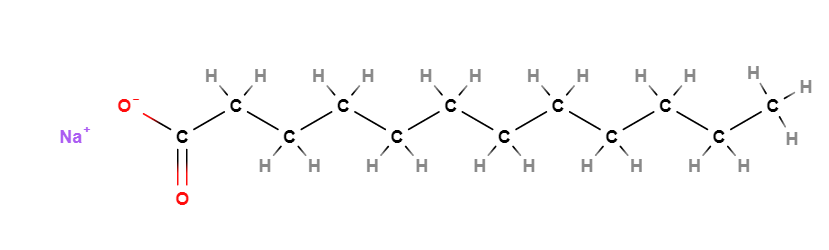 |  |
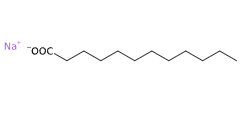
- Molecular Formula C12H23NaO2
- Linear Formula CH3(CH2)10COONa
- Molecular Weight 222.30
- Exact Mass 222.159576
- CAS 629-25-4
- UNII K146MR5EXO
- EC Number 211-082-4
- DSSTox Substance ID DTXSID9044453
- IUPAC sodium;dodecanoate
- InChI=1S/C12H24O2.Na/c1-2-3-4-5-6-7-8-9-10-11-12(13)14;/h2-11H2,1H3,(H,13,14);/q;+1/p-1
- InChl Key BTURAGWYSMTVOW-UHFFFAOYSA-M
- SMILES CCCCCCCCCCCC(=O)[O-].[Na+]
- MDL number MFCD00041754
- PubChem Substance ID 24896462
- ChEBI 131839
- RTECS OF0700000
- Beilstein 3574124
- NACRES NA.25
Synonyms
- Sodium dodecanoate
- Dodecanoic acid sodium salt
| Evaluate |

