| "Descrizione" by CarPas (5242 pt) | 2022-Aug-15 12:36 |
Review Consensus: 10 Rating: 10 Number of users: 1
| Evaluation | N. Experts | Evaluation | N. Experts |
|---|---|---|---|
| 1 | 6 | ||
| 2 | 7 | ||
| 3 | 8 | ||
| 4 | 9 | ||
| 5 | 10 |
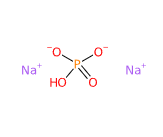
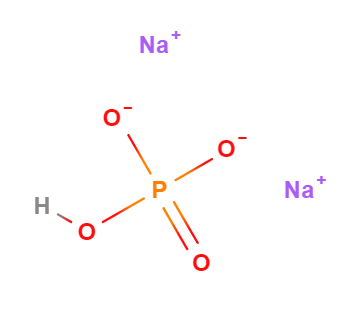
Dibasic sodium phosphate anhydrous or Sodium dibasic phosphate anhydrous is a chemical compound, one of the hydrated salts of sodium acid generated from phosphoric acid.
It appears as a white powder, soluble in water and the aqueous solution is weakly alkaline.

What it is used for and where
Medical
Together with Monobasic Sodium Phosphate, it is used in both adults and children in evacuative solutions such as disposable enemas for the treatment of constipation, as a pH regulator and saline laxative, for the treatment of acute and chronic constipation and also for colon cleansing as a preparation for surgical procedures (1). These chemical compounds increase water content and stool volume, creating a rectal distension effect. The required effect is usually produced within about 5 minutes.
Dibasic sodium phosphate is also used as therapy in lead poisoning.
Dentistry
The incorporation of dibasic sodium phosphate as a buffering agent in the composition of the mineral trioxide aggregate is recognised as one of the solutions to improve its properties, reduce time and achieve a diametral tensile strength of 4.9 MPa at the initial 6-hour period.(2). Nucleating agent.
Human nutrition
The addition of dibasic sodium phosphate to meat increases the pH value and ionic strength of the meat, improving water retention and the overall yield of the final product.
Baking agent to prevent oxidation, emulsifier
Animal feed
Dibasic sodium phosphate is used for oral phosphorus supplementation in animal feed. In Qianbei-Pockmarked goats suffering from a disorder referred to as 'Ruanguzheng's disorder', disodium hydrogen phosphate supplementation prevented and cured the disorder (3).
For more information:
Disodium hydrogen phosphate studies
Typical commercial product characteristics Sodium Phosphate, Dibasic
| Appearance | White crystal or powder, |
| pH | 8.8~9.2 |
| Boiling Point | 158ºC at 760 mmHg |
| Melting Point | 243-245°C |
| Density | 1.064 g/mL at 20°C |
| PSA | 93.23000 |
| Vapor density | 4.9 |
| Water Solubility | >=10 g/100 mL at 20ºC |
| Water insoluble | ≤ 0.4% |
| Loss on drying | ≤ 5.0% |
| Fluoride | ≤0.002% |
| Chloride | ≤ 0.06% |
| Sulphate | ≤ 0.2% |
| Arsenic | ≤ 0.0003% |
| Heavy Metals (As Pb) | ≤ 0.001% |
 |  |
 |  |
Price
1 kg €168.00
12 kg €1,010.00
- Molecular Formula HO4PNa2 o HNa2O4P o Na2HPO4
- Molecular Weight 141.959
- Exact Mass 141.940781
- CAS 7558-79-4
- UNII 22ADO53M6F
- EC Number 231-448-7
- DSSTox Substance ID DTXSID1026039
- IUPAC disodium;hydrogen phosphate
- InChI=1S/2Na.H3O4P/c;;1-5(2,3)4/h;;(H3,1,2,3,4)/q2*+1;/p-2
- InChl Key BNIILDVGGAEEIG-UHFFFAOYSA-L
- SMILES OP(=O)([O-])[O-].[Na+].[Na+]
- MDL number MFCD00003496
- PubChem Substance ID 329824633
- ChEBI 34683
- FEMA 2398
- ICSC 1129
- RTECS WC4500000
- UN 3082
- eCl@ss 38070211
- NACRES NA.21
Synonyms
- Disodium Phosphate Dibasic
- Disodium hydrogenorthophosphate
- Disodium hydrogen phosphate
- Sodium phosphate dibasic
- DSHP
References_____________________________________________________________
(1) Osgard E, Jackson JL, Strong J. A randomized trial comparing three methods of bowel preparation for flexible sigmoidoscopy. Am J Gastroenterol. 1998 Jul;93(7):1126-30. doi: 10.1111/j.1572-0241.1998.00342.x
(2) Ghasemi N, Rahimi S, Lotfi M, Solaimanirad J, Shahi S, Shafaie H, Salem Milani A, Shakuie S, Zand V, Abdolrahimi M. Effect of Mineral Trioxide Aggregate, Calcium-Enriched Mixture Cement and Mineral Trioxide Aggregate with Disodium Hydrogen Phosphate on BMP-2 Production. Iran Endod J. 2014 Summer;9(3):220-4.
(3) Shen X, Chi Y, Huo B, Xiong K. Studies on phosphorus deficiency in the Qianbei-Pockmarked goat. Asian-Australas J Anim Sci. 2019 Jun;32(6):896-903. doi: 10.5713/ajas.18.0622.
| Evaluate |

