| "Descrizione" by Street82 (2970 pt) | 2023-Feb-15 09:58 |
Review Consensus: 10 Rating: 10 Number of users: 1
| Evaluation | N. Experts | Evaluation | N. Experts |
|---|---|---|---|
| 1 | 6 | ||
| 2 | 7 | ||
| 3 | 8 | ||
| 4 | 9 | ||
| 5 | 10 |
Ricinus communis seed oil is a vegetable oil obtained by cold pressing or other methods of extraction (chemical or solvent extraction using n-hexane and supercritical carbon dioxide, aqueous and reactive enzymatic accompanied by ultrasound) of the seeds of Ricinus communis L., (oil content approximately 35%-56%) an annual herbaceous plant belonging to the Euphorbiaceae family. It is composed of ricinoleic acid, a hydroxylated fatty acid, carotenoids, tocopherols and other interesting constituents.
It appears as a white powder or a viscous, transparent yellowish liquid. May be sensitive to light. Flammable. Incompatible with strong oxidising agents. Insoluble in water, insoluble in mineral oil unless mixed with another vegetable oil. Mixable with ethanol, chloroform, diethylether, acetic acid and methanol.


What it is used for and where
Medical
Castor oil has been used by traditional medical science as a purgative and as a method of initiating labour in obstetrical practice. Ricinus communis contains 80-90% ricinoleic acid and, among other applications, is used as a co-polyester of ricinoleic-lactic acid as a drug carrier (1) and has demonstrated anti-inflammatory, bactericidal and anti-herpetic properties (2).
Cosmetics
At the origin of synthetic surfactants Turkey Red Oil was the first on the market and was and still is the sulphated product of castor oil, still used today in the processing and tanning of leather, textiles and in some cosmetic applications.
Castor oil is added to cold-processed soap formulations to increase lather, added to shampoos and conditioners to treat brittle hair and add volume. Its high viscosity and miscibility with both polar and non-polar compounds make it especially interesting added in small amounts in lipsticks. It has a non-comedogenic skin interaction so it does not create unwanted films on the skin and this quality is the reason why it is popular in skin-improving creams. Cleansers containing castor oil have emulsifying properties.
Skin conditioning agent. An ingredient that is the mainstay of topical skin treatment by restoring, increasing or improving skin tolerance to external factors, including melanocyte tolerance. The most important function of the conditioning agent is to prevent skin dehydration, but the subject is rather complex and involves emollients and humectants.
Fragrance. It plays a very important role in the formulation of cosmetic products as it allows perfume to be enhanced, masked or added to the final product, improving its commercial viability. The consumer always expects to find a pleasant scent in a cosmetic product.
In powder form it is hydrogenated and is an emulsifying agent.

Insecticides
Castor oil has shown some potential in the practical control of both immature and adult stages of the mosquito vector, particularly against the important dengue vector, Aedes aegypti, and has been tested for macrophilicidal activity against adults of Setaria digitata a parasitic nematode found in the peritoneal cavity of cattle. The toxicity of methanol, ethyl acetate, chloroform and petroleum ether extracts of the leaf of R. communis was tested against third stage Musca domestica larvae (Singh & Kaur, 2016). Crude methane extracts of R. communis leaves showed moderate acaricidal and insecticidal activity against Haemaphysalis bispinosa and Hippobosca maculate (Zahir et al., 2010a) (3).
Other Uses
Used to produce polyurethane coatings, rubber, dicarboxylic acid, plasticisers and various types of oil such as insulating oil, Turkish red oil, hydraulic oil. It is also used to produce polyamide-11 fibre and soap.
In the leather industry it serves as a greasing agent, extender and bleaching agent for finishing.
In the textile industry it is a penetrating and emulsifying agent.
When dehydrated, a conjugated dry double bond oil is obtained.
Safety
In 2007, the CIR (Cosmetic Ingredient Review) Expert Group suggested that sensitisation reactions were rarely observed and this ingredient can be used safely in cosmetic products even when aerosolised because the particle sizes produced are not respirable. Overall, the CIR Expert Panel concluded that this cosmetic ingredient is safe in the use practices and concentrations described in this safety assessment (4).
For more information:
Typical commercial product characteristics Castor seed oil
| Appearance | White powder or colorless or slight yellow liquid |
| Boiling Point | 879.2±65.0°C at 760 mmHg |
| Melting Point | -12ºC |
| Flash Point | 224.1±27.8°C |
| Freezing Point | -10℃ |
| Density | 1.0±0.1 g/cm3 |
| Relative density | 0.956 ~ 0.969 |
| Refraction Index | 1.490 |
| Vapor Pressure | 0.0±0.6 mmHg at 25°C |
| PSA | 139.59000 |
| LogP | 17.72 |
| Water Solubility | <0.1 g/100 mL at 20 ºC |
| Refractive Rate | n20/D1.478(lit.) |
| Autogenous Ignition | 449°C (liquid) |
| Relative density | 0.956 ~ 0.969 (liquid) |
| Shelf Life | 24 months |
 |  |
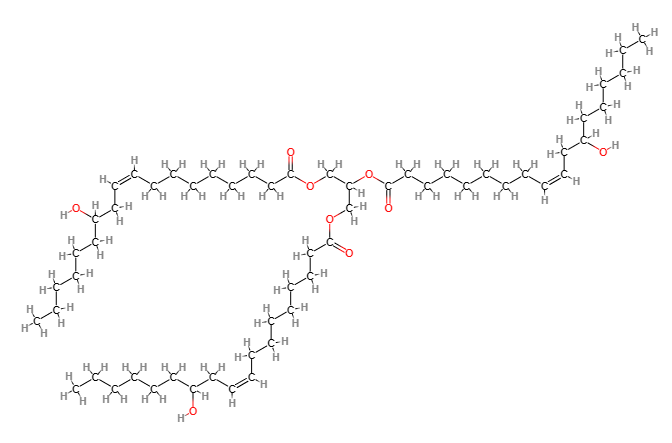 |  |
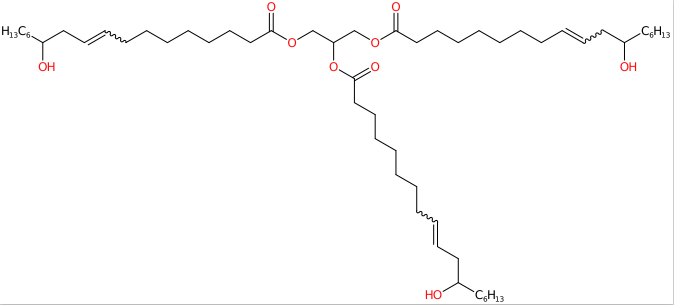
- Molecular Formula C57H104O9
- Molecular Weight 933.4
- Exact Mass 932.768005
- CAS 8001-79-4
- UNII D5340Y2I9G
- EC Number 232-293-8
- DSSTox Substance ID DTXSID7024742
- IUPAC 2,3-bis[[(Z)-12-hydroxyoctadec-9-enoyl]oxy]propyl (Z)-12-hydroxyoctadec-9-enoate
- InChl=1S/C57H104O9/c1-4-7-10-31-40-51(58)43-34-25-19-13-16-22-28-37-46-55(61)64-49-54(66-57(63)48-39-30-24-18-15-21-27-36-45-53(60)42-33-12-9-6-3)50-65-56(62)47-38-29-23-17-14-20-26-35-44-52(59)41-32-11-8-5-2/h25-27,34-36,51-54,58-60H,4-24,28-33,37-50H2,1-3H3/b34-25-,35-26-,36-27-
- InChl Key ZEMPKEQAKRGZGQ-AAKVHIHISA-N
- SMILES CCCCCCC(CC=CCCCCCCCC(=O)OCC(COC(=O)CCCCCCCC=CCC(CCCCCC)O)OC(=O)CCCCCCCC=CCC(CCCCCC)O)O
- MDL number MFCD00130746
- PubChem Substance ID
- RTECS FI4100000 YX1850000
- FEMA 2263
- NCI C80990
- ICSC 1452
Synonyms:
- Ricinus oil
- Castor oil
- Olio di ricino
References_______________________________________________________________________
(1) Slivniak, R.; Ezra, A.; Domb, A.J. Hydrolytic degradation and drug release of ricinoleic acid-lactic acid copolyesters. Pharm. Res. 23, 1306-1312 (2006)
(2) Gamayurova, V.S.; Zinov’Eva, M.E.; Tran, H.T.T. Features of the enzymatic hydrolysis of castor oil. Catal. Ind. 5, 269-273 (2013).
(3) Wamaket N, Dieng H, Komalamisra N, Apiwathnasorn C, Morales RE, Thanomsub BW, Srisawat R, Attrapadung S. Larvicidal and adulticidal activities of castor oil against the dengue vector, Aedes aegypti. Trop Biomed. 2018 Sep 1;35(3):610-618.
(4) Final report on the safety assessment of Ricinus Communis (Castor) Seed Oil, Hydrogenated Castor Oil, Glyceryl Ricinoleate, Glyceryl Ricinoleate SE, Ricinoleic Acid, Potassium Ricinoleate, Sodium Ricinoleate, Zinc Ricinoleate, Cetyl Ricinoleate, Ethyl Ricinoleate, Glycol Ricinoleate, Isopropyl Ricinoleate, Methyl Ricinoleate, and Octyldodecyl Ricinoleate. Int J Toxicol. 2007;26 Suppl 3:31-77. doi: 10.1080/10915810701663150.
| Evaluate |

