| "Descrizione" by AColumn (9336 pt) | 2023-Jun-21 11:03 |
Review Consensus: 10 Rating: 10 Number of users: 1
| Evaluation | N. Experts | Evaluation | N. Experts |
|---|---|---|---|
| 1 | 6 | ||
| 2 | 7 | ||
| 3 | 8 | ||
| 4 | 9 | ||
| 5 | 10 |
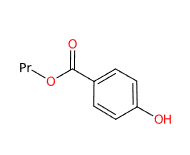
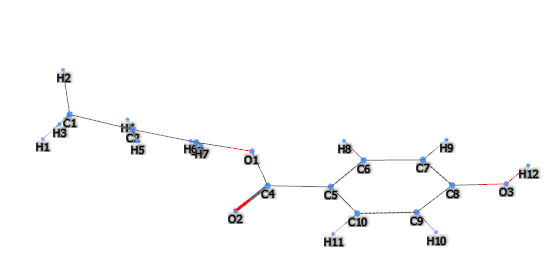
Propyl-p-hydroxybenzoate (Propylparaben) is a chemical compound, benzoate propyl ester of 4-hydroxybenzoic acid, and belongs to the chemical family of parabens.
Security. Foreword
Although this allergen is less and less used, warning must be given because it is labelled under different nomenclature, with different names corresponding to its many synonyms.
In 2014, the European Union banned the use of iso-propyl paraben, iso-butyl paraben, benzyl paraben, and pentyl paraben in cosmetics and iso-propyl paraben in food products, while the use of methylparaben and ethylparaben (EtP) is limited to a maximum of 0.4% per single compound and 0.8% per mixture of esters. o single ester.
In this study on the elderly population in the United States, exposure to parabens was positively associated with low cognitive performance in older male subjects (1) and with oestrogenic activity (e.g. water retention) and therefore being evaluated as endocrine disrupters (2).
The name describes the structure of the molecule:
- Propyl refers to a propyl group, which is a three carbon atom alkyl substituent with formula -C3H7.
- p-hydroxybenzoate refers to a benzoate group derived from benzoic acid with a hydroxy group (-OH) attached to the para position of the benzene ring. Benzoate is the ester of benzoic acid and has the structure -C6H5COO.
Industrially it appears as a white crystalline powder. Stable, incompatible with strong oxidising agents and strong bases.

What it is used for and where
Food
Antimicrobial preservative. It is labelled with the number E216 in the list of European food additives as a preservative and serves to fight bacteria, fungi, yeasts and moulds, however, it has been found in numerous clinical studies to have an endocrine disrupting effect. Although this allergen is used less and less, warning must be given because it is written on labels under different nomenclature, with different names corresponding to its many synonyms.
- Doses normally used in food products
- fresh fruit and vegetables 0.012%.
- Fruit juices (fruity), jam (excluding tins), soy sauce, 0.25%
- Confectionery filling, 0.5% (total amount disposable or mixed with p-hydroxybenzoic ethyl ester)
- Egg yolk filling 0.20%.
So beware of cumulative intake.
Cosmetics
It is a restricted ingredient as V/12 a Relevant Item in the Annexes of the European Cosmetics Regulation 1223/2009.
Preservative. Any product containing organic, inorganic compounds, water, needs to be preserved from microbial contamination. Preservatives act against the development of harmful microorganisms and against oxidation of the product.
Antimicrobial preservative belonging to the paraben family, a class of chemical compounds to which the scientific literature attributes endocrine disrupting characteristics. Although this allergen is less and less used, warning must be given because it is written on labels under different nomenclature, with different names corresponding to its many synonyms. It is currently regulated as a preservative in concentrations up to 0.14% (as an acid) and 'the current level of evidence is insufficient to consider it an endocrine disruptor or to derive a toxicological starting point based on its endocrine disrupting properties' (3) and so far the experts' study appears reassuring, however the overall cumulative intake of parabens for those who use many creams or body care products containing parabens must be considered. The endocrine system hinges on the interactive balancing act between hormones, and disruption of this delicate balance can lead to increased intracellular oestrogen levels in adipocytes, in effect enhancing obesity throughout the body or in certain parts of the body. Clinical studies recommend avoiding exposure to endocrine disruptors to reduce weight (Darbre 2017). Parabens can enter the human body through ingestion, absorption through the skin or inhalation. And this is where cumulation can come into play.
Perfuming. Unlike fragrance, which can also contain slightly less pleasant or characteristic odours, the term perfume indicates only very pleasant fragrances. Used for perfumes and aromatic raw materials.
Medical
The news about Propylparaben is not so worrying in medical terms, but has provided grounds for study for specific applications. At controlled doses this preservative has shown protective activity in severe traumatic brain injury (4) and could represent a new therapeutic strategy in drug-resistant spontaneous recurrent seizures of the epileptic state (5).
The most relevant studies and their abstracts have been selected to explore this in more depth:
Propyl-p-hydroxybenzoate studies
| Appearance | White powder |
| Boiling Point | 294.3±13.0°C at 760 mmHg |
| Melting Point | 95-98°C |
| Flash Point | 124.6±12.6°C |
| Density | 1.1±0.1 g/cm3 |
| Refraction Index | 1.532 |
| Vapor Pressure | 0.0±0.6 mmHg at 25°C |
| Water Solubility | <0.1 g/100 mL at 12ºC |
| PSA | 46.53000 |
| LogP | 2.93 |
| Loss on drying | ≤0.5% |
| Residue on ignition | ≤0.1% |
| Sulphated Ash | 0.1% max |
| Sulfate So42- | 0.024%Max |
| Residue | 0.05% max |
| Heavy Metal | 10 ppm max |
| Acidity | <0.1 |
| P-hydroxybenzoic acid | ≤0.50% |
| Single impurities | ≤0.50% |
| Heavy Metal Pb | ≤1.0% |
| Specific Gravity | 0.789 (20/4℃) |
| Storage | 0-6°C |
 |  |
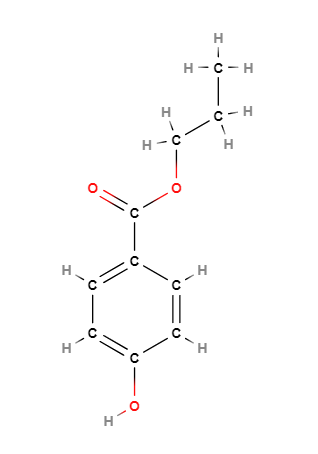 |  |
- Molecular Formula C10H12O3
- Linear Formula HOC6H4CO2CH2CH2CH3
- Moleculart Weight 180.203 g/mol
- Exact Mass 180.078644
- CAS 94-13-3
- UNII Z8IX2SC1OH
- EC Number 202-307-7
- DSSTox Substance ID DTXSID4022527
- IUPAC propyl 4-hydroxybenzoate
- InChl=1S/C10H12O3/c1-2-7-13-10(12)8-3-5-9(11)6-4-8/h3-6,11H,2,7H2,1H3
- InChl Key QELSKZZBTMNZEB-UHFFFAOYSA-N
- SMILES CCCOC(=O)C1=CC=C(C=C1)O
- MDL number MFCD00002354
- PubChem Substance ID 329823041
- ChEBI 32063
- RTECS DH2800000
- NCI C76730
- RXCUI 34706
- NSC 23515 8511
- FEMA Number 2951
- Beilstein 1103245
- NACRES NA.24
Synonyms:
- 4-Hydroxybenzoic acid propyl ester
- Propyl p-hydroxybenzoate
- Propyl 4-hydroxybenzoate
- Benzoic acid, 4-hydroxy-, propyl ester
- p-Hydroxybenzoic acid n-propyl ester
- Nipasol
- Nipazol
- E216
References________________________________________________________________________
(1) Shi Y, Wang H, Zhu Z, Ye Q, Lin F, Cai G. Association between exposure to phenols and parabens and cognitive function in older adults in the United States: A cross-sectional study. Sci Total Environ. 2022 Nov 9:160129. doi: 10.1016/j.scitotenv.2022.160129.
(2) Aline Murawski, Carolin Tschersich, Enrico Rucic, Gerda Schwedler, Rebecca K. Moos, Monika Kasper-Sonnenberg, Thomas Brüning, Holger M. Koch, Marike Kolossa-Gehring, Parabens in urine of children and adolescents in Germany – human biomonitoring results of the german environmental survey 2014–2017 (GerES V), Environmental Research, Volume 194, 2021, 110502, ISSN 0013-9351, https://doi.org/10.1016/j.envres.2020.110502. (https://www.sciencedirect.com/science/article/pii/S0013935120313992) Abstract: Parabens are antimicrobial preservatives used in a wide range of consumer products such as personal care products, cosmetics, pharmaceuticals, and food. Consequently, the general population is ubiquitously exposed to these substances via dermal absorption, ingestion, and inhalation. Parabens promote estrogenic activity and are hence under assessment as endocrine disrupting substances. Urine samples from 3- to 17-year-old children and adolescents (N = 516) living in Germany were analysed for concentrations of nine parabens in the population representative German Environmental Survey for Children and Adolescents 2014–2017 (GerES V). Detection rates and urinary concentrations of the parabens decreased with increasing length of the alkyl chain. Methyl paraben was quantified in 97% of the samples with a geometric mean (GM) concentration of 7.724 μg/L (6.714 μg/gcreatinine), ethyl paraben was quantified in 69% (GM: 0.943 μg/L and 0.825 μg/gcrea), and n-propyl paraben in 31% (GM: 0.563 μg/L and 0.493 μg/gcrea). Concentrations of iso-propyl paraben, butyl paraben, iso-butyl paraben, and benzyl paraben were below the limit of quantification in most samples. Pentyl paraben and heptyl paraben were not detected in any of the samples. Paraben concentrations in urine were found to be associated with frequent usage of leave-on personal care products and cosmetics. Cumulative exposure to parabens (back-calculated daily intakes, expressed as hazard index) was found to be on a level raising concern in up to 14% of the population, mainly driven by n-propyl paraben, and depending on the level of conservativeness and point-of departures used for calculation.
(3) SCCS Members. Electronic address: SANTE-C2-SCCS@ec.europa.eu; SCCS External Experts; SCCS Members. Opinion of the Scientific Committee on Consumer Safety (SCCS) - Final Opinion on propylparaben (CAS No 94-13-3, EC No 202-307-7). Regul Toxicol Pharmacol. 2021 Oct;125:105005. doi: 10.1016/j.yrtph.2021.105005.
(4) Santiago-Castañeda C, Segovia-Oropeza M, Concha L, Orozco-Suárez SA, Rocha L. Propylparaben Reduces the Long-Term Consequences in Hippocampus Induced by Traumatic Brain Injury in Rats: Its Implications as Therapeutic Strategy to Prevent Neurodegenerative Diseases. J Alzheimers Dis. 2021;82(s1):S215-S226. doi: 10.3233/JAD-200914.
(5) Santana-Gómez CE, Valle-Dorado MG, Domínguez-Valentín AE, Hernández-Moreno A, Orozco-Suárez S, Rocha L. Neuroprotective effects of levetiracetam, both alone and combined with propylparaben, in the long-term consequences induced by lithium-pilocarpine status epilepticus. Neurochem Int. 2018 Nov;120:224-232. doi: 10.1016/j.neuint.2018.09.004.
| Evaluate |

