| "Descrizione" by Ark90 (12432 pt) | 2024-Sep-24 19:37 |
Review Consensus: 10 Rating: 10 Number of users: 1
| Evaluation | N. Experts | Evaluation | N. Experts |
|---|---|---|---|
| 1 | 6 | ||
| 2 | 7 | ||
| 3 | 8 | ||
| 4 | 9 | ||
| 5 | 10 |
Alpha Isomethyl Ionone is a chemical compound obtained by a high-temperature chemical process. It is also known as Acetone or Methyl ionone.
The name describes the structure of the molecule:
- Alpha. This term is often used in organic chemistry to indicate the location of a functional or substituent group. In this case, it refers to the location of the isomethyl group in the ionon molecule.
- Isomethyl refers to an isomer of a methyl group. An isomer is a compound with the same molecular formula as another compound but a different arrangement of atoms in space.
- Ionone. This is a term used to describe a variety of fragrant substances found in a wide range of essential oils. Ionones are often used in perfumery and cosmetics for their pleasant scent.
Description of raw materials used in production:
- Citral - An organic compound used as a starting point in the synthesis of ionones. It's present in many essential oils and has a lemon-like fragrance.
- Hydrogen and catalysts - Used for reducing citral to ionones.
The synthesis process takes place in several stages:
- Preparation. Methylheptenone can be synthesized by reacting isoprene with Methacrolein in the presence of an acid catalyst.
- Cyclization. Methylheptenone is cyclized, using an acid catalyst, to form a mixture of isomeric ionons, including the alpha-isomethyl ion.
It appears as a colourless, white to light yellow liquid with a typical violet odour.


What it is used for and where
Cosmetics
Alpha-Isomethyl Ionone is a restricted ingredient as III/90 a Relevant Item in the Annexes of the European Cosmetics Regulation 1223/2009.
It is a chemical fragrance that is added to products such as liquid soaps, shampoos and others to add a violet scent.
- Skin conditioning agent. It is the mainstay of topical skin treatment as it has the function of restoring, increasing or improving skin tolerance to external factors, including melanocyte tolerance. The most important function of the conditioning agent is to prevent skin dehydration, but the subject is rather complex and involves emollients and humectants that can be added in the formulation.
- Perfuming. Unlike fragrance, which can also contain slightly less pleasant or characteristic odours, the term perfume indicates only very pleasant fragrances. Used for perfumes and aromatic raw materials.

It is a product that can give allergies.
Other uses
Used as a cold-resistant plasticiser, fixative for gas chromatography, main plasticiser in plastics.
More information:
"Alpha-Isomethyl Ionone, studies"
Typical commercial product characteristics Alpha-Isomethyl Ionone
| Appearance | Colorless or Yellow liquid |
| Boiling Point | 285.3±29.0°C at 760 mmHg |
| Flash Point | 122.1±17.5°C |
| Density | 0.9±0.1 g/cm3 |
| Vapor Pressure | 0.0±0.6 mmHg at 25°C |
| Refraction Index | 1.508 |
| Specific Gravity | 0.925 |
| PSA | 17.07000 |
| LogP | 4.41 |
| Storage | Room Temperature |
| Chemical Safety |  |
 |  |
 |  |
Price:
50ml/1.70 oz $14.50
1000ml/33.80oz $208.00
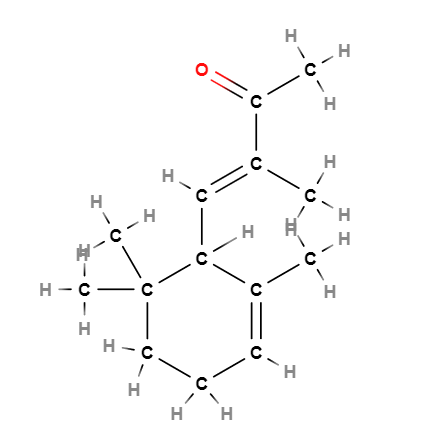
- Molecular Formula C14H22O
- Molecular Weight 206.32
- Exact Mass 206.167068
- CAS 127-51-5
- UNII 9XP4LC555B
- EC Number 204-846-3
- DSSTox Substance ID DTXSID7027047 DTXSID10859248
- IUPAC (E)-3-methyl-4-(2,6,6-trimethylcyclohex-2-en-1-yl)but-3-en-2-one
- InChl=1S/C14H22O/c1-10-7-6-8-14(4,5)13(10)9-11(2)12(3)15/h7,9,13H,6,8H2,1-5H3/b11-9+
- InChl Key JRJBVWJSTHECJK-PKNBQFBNSA-N
- SMILES CC1=CCCC(C1C=C(C)C(=O)C)(C)C
- MDL number MFCD00034582
- PubChem Substance ID 329751380
- ChEBI 179835
- SCHEMBL 891912
- NSC 66432
- FEMA 2714
- JECFA 404
- NACRES NA.24
- HS Code 2914299000
Synonyms :
- 3-methyl-4-(2,2-dimethyl-6-methylenecyclohexyl)-3-buten-2-one
- 4-(2,6-Trimethyl-2-cyclohexen-1-yl)-3-methyl-3-buten-2-one
- Isoraldeine
- Methyl ionone
- Alpha-cetone
- Methyl ionone gamma
- Acetone 99,5%
- α-Cetone
- α−Isomethyl ionone
- Iso-.alpha.-methyl ionone
- Gamma Methyl Ionone
- AC1NS9PL
References__________________________________________________________________________
Yamazaki Y, Hayashi Y, Arita M, Hieda T, Mikami Y. Microbial Conversion of alpha-Ionone, alpha-Methylionone, and alpha-Isomethylionone. Appl Environ Microbiol. 1988 Oct;54(10):2354-60. doi: 10.1128/aem.54.10.2354-2360.1988. PMID: 16347747; PMCID: PMC204258.
Abstract. alpha-Ionone, alpha-methylionone, and alpha-isomethylionone were converted by Aspergillus niger JTS 191. The individual bioconversion products from alpha-ionone were isolated and identified by spectrometry and organic synthesis. The major products were cis-3-hydroxy-alpha-ionone, trans-3-hydroxy-alpha-ionone, and 3-oxo-alpha-ionone. 2,3-Dehydro-alpha-ionone, 3,4-dehydro-beta-ionone, and 1-(6,6-dimethyl-2-methylene-3-cyclohexenyl)-buten-3-one were also identified. Analogous bioconversion products from alpha-methylionone and alpha-isomethylionone were also identified. From results of gas-liquid chromatographic analysis during the fermentation, we propose a metabolic pathway for alpha-ionones and elucidation of stereochemical features of the bioconversion.
Avonto C, Wang M, Chittiboyina AG, Vukmanovic S, Khan IA. Chemical stability and in chemico reactivity of 24 fragrance ingredients of concern for skin sensitization risk assessment. Toxicol In Vitro. 2018 Feb;46:237-245. doi: 10.1016/j.tiv.2017.09.007.
An S, Lee AY, Lee CH, Kim DW, Hahm JH, Kim KJ, Moon KC, Won YH, Ro YS, Eun HC. Fragrance contact dermatitis in Korea: a joint study. Contact Dermatitis. 2005 Dec;53(6):320-3. doi: 10.1111/j.0105-1873.2005.00720.x.
Abstract. The purpose of this study is to determine the frequency of responses to selected fragrances in patients with suspected fragrance allergy and to evaluate the risk factors. 9 dermatology departments of university hospitals have participated in this study for the past 1 year. To determine allergic response to fragrances, 18 additional fragrances in addition to the Korean standard and a commercial fragrance series were patch-tested in patients with suspecting cosmetic contact dermatitis. Over 80% of the patients were women, and the most common site was the face. Cinnamic alcohol and sandalwood oil (Santalum album L.) showed high frequencies of positive responses. Of the specific fragrances, ebanol, alpha-isomethyl-ionone (methyl ionone-gamma) and Lyral (hydroxyisohexyl 3-cyclohexane carboxdaldehyde) showed high positive responses. We compared the results obtained during this study with those of other studies and concluded that including additional fragrance allergens may be useful for the detection of fragrance allergy.
Ishizaki S, Itoh M, Komai T, Honda T, Kitahara T. Biotransformation of alpha-isomethylionone to 1-(2,6,6-trimethyl-2-cyclohexen-1-yl)propan-2-one. Biosci Biotechnol Biochem. 2004 May;68(5):1164-6. doi: 10.1271/bbb.68.1164.
Abstract. The main biodegradation product of (+/-)-alpha-isomethylionone (2) with standard activated sludge was characterized as (+/-)-1-(2,6,6-trimethyl-2-cyclohexen-1-yl)propan-2-one (1) by its analysis and synthesis. Both enantiomers (1a and 1b) of 1 were synthesized by starting from (R)- and (S)-2,4,4-trimethyl-2-cyclohexen-1-ol (3a and 3b), respectively.
| Evaluate |

