| "Descrizione" by Whiz35 (11840 pt) | 2023-Jul-06 11:58 |
Review Consensus: 10 Rating: 10 Number of users: 1
| Evaluation | N. Experts | Evaluation | N. Experts |
|---|---|---|---|
| 1 | 6 | ||
| 2 | 7 | ||
| 3 | 8 | ||
| 4 | 9 | ||
| 5 | 10 |
Ethanolamine (MEA, Monoethanolamine, 2-aminoethanol) is a chemical compound belonging to the first generation of ethanolamines, is a primary amine and is a strong alkaline agent, corrosion inhibitor and chemical intermediate and acts as both an amine and an alcohol.
The name defines the structure of the molecule:
- Ethanol- refers to the ethanol portion of the carbon of the molecule, which includes a hydroxyl (-OH) group.
- -amine indicates the presence of an amino group (-NH2) in the molecule.
The monoethanolamine synthesis process takes place in several stages:
- Preparation of raw materials: ethylene oxide and ammonia. Ethylene oxide is a cyclic ether and is highly reactive due to the tension in the three-member ring. Ammonia is a weak base that can react with ethylene oxide.
- Reaction. The reaction between ethylene oxide and ammonia takes place in a reactor. Ethylene oxide is added to ammonia under controlled conditions of temperature and pressure. The reaction is exothermic, that is, it releases heat.
- Purification.The reaction mixture is purified to remove any raw materials and unreacted by-products through a distillation process, where the mixture is heated and the different components are separated according to their boiling points.
- Quality control. The final product is tested to ensure that it meets the required specifications by verifying the pH, density and purity of monoethanolamine.
It appears as a colourless liquid.
Safety. MEA should not be included in products formulated as aerosols and in products containing N-nitroso. Scientific literature agrees that MEA has the ability to penetrate and absorb into the skin and cause skin irritation, especially on the scalp (1).
What it is used for and where
It is one of the most common amines, used for years to absorb hydrogen sulphide and carbon dioxide from natural gases.
Cosmetics
It is a restricted ingredient as III/61 a Relevant Item in the Annexes of the European Cosmetics Regulation 1223/2009. Ingredient at risk: Monoalkylamines, monoalkanolamines and their salts
- Buffering agent. It is an iingredient that can bring an alkaline or acid solution to a certain pH level and prevent it from changing, in practice a pH stabiliser that can effectively resist instability and pH change.
Hair conditioning agent. A large number of ingredients with specific purposes can co-exist in a hair shampoo: cleansers, conditioners, thickeners, mattifying agents, sequestering agents, fragrances, preservatives, special additives. However, the indispensable ingredients are the cleansers and conditioners as they are necessary and sufficient for hair cleansing and manageability. The others act as commercial and non-essential auxiliaries such as: appearance, fragrance, colouring, etc. Hair conditioning agents have the task of increasing shine, manageability and volume, and reducing static electricity, especially after treatments such as colouring, ironing, waving, drying and brushing. They are, in practice, dispersing agents that may contain cationic surfactants, thickeners, emollients, polymers. The typology of hair conditioners includes: intensive conditioners, instant conditioners, thickening conditioners, drying conditioners.
pH adjuster. This ingredient tends to restore the pH of a cosmetic formulation to its optimal value. The correct pH value is an essential determinant for lipid synthesis in the stratum corneum. The average physiological pH value of the face ranges between 5.67 and 5.76. The hair fibre has a pH value of 3.67.
Skin conditioning agent - Miscellaneous. Ingredient that has the task of modifying the condition of the skin when it is damaged or dry by reducing its flakiness and restoring its elasticity.
Surfactant - Foam booster. Its function is to introduce gas bubbles into the water for a purely aesthetic factor, which does not affect the cleaning process, but only satisfies the commercial aspect of the detergent by helping to spread the detergent on the hair. This helps in the commercial success of a shampoo formulation. Since sebum has an inhibiting action on the bubble, more foam is produced in the second shampoo.
Other uses
- Chemical reagent
- Corrosion inhibitor
- Gas chromatographic liquid
- Solvent
- Lightening agent for printing and dyeing
- Fabric mothproofing agent
The most relevant studies on this ingredient have been selected with a summary of their contents:
Typical commercial product characteristics MEA
| Appearance | Transparent colorless liquid |
| Boiling Point | 170.9±0.0 °C at 760 mmHg |
| Melting Point | 10-11 °C(lit.) |
| Flash Point | 93.3±0.0 °C |
| Density | 1.0±0.1 g/cm3 |
| Refraction Index | 1.435 |
| Vapor Pressure | 0.5±0.7 mmHg at 25°C |
| Vapor density | 2.1 |
| PSA | 46.25000 |
| LogP | -1.31 |
| Monoethanolamine | 99.7 ω/% |
| Diethanolamine | 0.1 ω/% |
| Distillate volume | 95 at 168~174℃ |
| Chemical Safety | 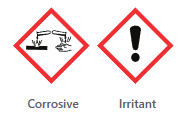 |
 |  |
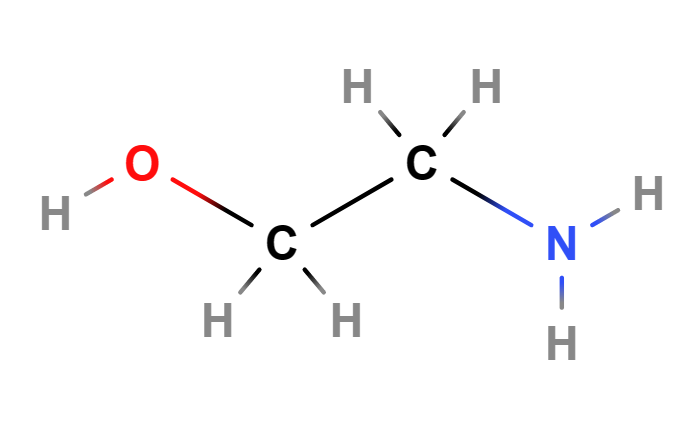 | 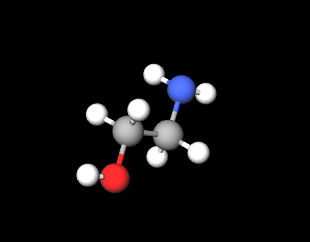 |
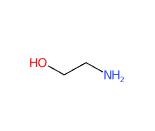
- Molecular Formula H2NCH2CH2OH C2H7NO
- Molecular Weight 61.08
- Exact Mass 61.052765
- CAS 141-43-5
- UNII 5KV86114PT
- EC Number 205-483-3
- DSSTox Substance ID DTXSID6022000
- IUPAC 2-aminoethanol
- InChl=1S/C2H7NO/c3-1-2-4/h4H,1-3H2
- InChl Key HZAXFHJVJLSVMW-UHFFFAOYSA-N
- SMILES C(CO)N
- MDL number MFCD00008183
- PubChem Substance ID 329799085
- ChEBI 16000
- Beilstein 505944
- RTECS KJ5775000
- NCI C61756
- ICSC 0152
- NACRES NA.25
- UN 2491
- RXCUI 24457
Synonyms:
- 2-Hydroxyethylamine
- 1-amino-2-hydroxy-ethane
- aminomethylpropanol
- 2-Aminoethanol,2-Aminoethyl alcohol,ETA
- 1-Amino-2-butanol
- MEA
- Ethanolamine
- amino-2-butanol
- mono-sec-butanolamine
- 2-Butanol,1-amino
References____________________________________________________________________
(1) Geier J, Lessmann H, Dickel H, Frosch PJ, Koch P, Becker D, Jappe U, Aberer W, Schnuch A, Uter W. Patch test results with the metalworking fluid series of the German Contact Dermatitis Research Group (DKG). Contact Dermatitis. 2004 Sep;51(3):118-30. doi: 10.1111/j.0105-1873.2004.00416.x.
| Evaluate |

