Check the ingredients!
... live healthy!


| "Descrizione" by Al222 (19776 pt) | 2024-Oct-05 18:30 |
Solvent Yellow 1, also known as 4-Aminoazobenzene, is a synthetic azo dye that provides a yellow to orange hue. It is primarily used in industrial applications such as coloring plastics, oils, waxes, and fuels due to its stability and solubility in organic solvents. Historically, it was used in cosmetics, textiles, and food products, but its use in consumer items has been heavily restricted or banned in many countries due to concerns over its potential health risks, particularly its classification as a possible carcinogen.
Chemical Composition and Structure
Solvent Yellow 1 is an aromatic azo compound with the chemical formula C12H11N3. It consists of two benzene rings connected by an azo group (-N=N-), with an amine group (-NH2) attached to one of the benzene rings. The azo bond is responsible for the dye’s characteristic yellow color, and the aromatic structure gives the compound its stability. The presence of the amine group makes the compound reactive under certain conditions, leading to potential breakdown into aromatic amines, which are known to pose health risks.
Physical Properties
Solvent Yellow 1 appears as a yellow to orange crystalline powder. It is highly soluble in organic solvents such as alcohols, hydrocarbons, and oils, making it ideal for use in non-aqueous systems. However, it is insoluble in water, limiting its use in aqueous formulations. The dye is thermally stable and resistant to light degradation, which makes it suitable for applications where long-term color retention is important, such as in fuels and plastics. Its stability in the presence of heat and light helps maintain color consistency in industrial products.
Production Process
The production of 4-Aminoazobenzene (Solvent Yellow 1) involves the chemical synthesis of azo compounds through a process called diazotization, where an aromatic amine (such as aniline) is reacted with nitrous acid, creating a diazonium salt. This diazonium compound is then coupled with another aromatic amine or a phenolic compound, forming the azo bond (-N=N-). The process is closely monitored to ensure the purity and consistency of the final product. However, due to concerns over its breakdown into aromatic amines, production for consumer use is tightly regulated.
Applications
Industrial Use: Solvent Yellow 1 is widely used in industrial settings to color a variety of materials, including:
Historical Use in Cosmetics: Before regulatory changes, Solvent Yellow 1 was used in oil-based cosmetics, such as lipsticks, and personal care products for its vibrant color. However, its use in these applications has been banned due to health risks associated with prolonged exposure.
Textiles and Leather: Solvent Yellow 1 was also historically used in the textile and leather industries for dyeing fabrics and leather goods. However, its application in these fields has been largely phased out due to safety regulations.
Safety and Health Concerns
Carcinogenicity
Solvent Yellow 1 (4-Aminoazobenzene) has been classified as a potential carcinogen. The primary concern stems from its ability to break down into aromatic amines, specifically aniline, which are known to be carcinogenic. Studies have shown that long-term exposure to azo dyes, particularly those that can release aromatic amines, may increase the risk of cancer, particularly bladder cancer. As a result, the use of Solvent Yellow 1 in consumer products such as cosmetics, food, and textiles has been banned or heavily restricted in many countries, including those in the European Union, the United States, and Japan.

Toxicity
In addition to its carcinogenic potential, Solvent Yellow 1 can be toxic if ingested or inhaled in significant amounts. Direct contact with the skin may also cause irritation. Its potential to bioaccumulate in living organisms raises concerns about its long-term environmental and health impacts. For this reason, its use is largely limited to industrial settings with proper safety protocols in place to minimize exposure.
Environmental and Safety Considerations
Solvent Yellow 1 is not easily biodegradable and can persist in the environment, particularly in aquatic ecosystems. Its breakdown into harmful aromatic amines contributes to its environmental persistence and potential toxicity to aquatic organisms. Waste management and disposal of products containing Solvent Yellow 1 must be carefully controlled to prevent environmental contamination. Industrial facilities using this dye are required to follow strict environmental regulations to minimize its impact on ecosystems.
Regulatory Status
Due to its classification as a potential carcinogen, the use of Solvent Yellow 1 has been heavily regulated. In many countries, it is banned in food products, cosmetics, and textiles. The European Union’s REACH regulation and the U.S. FDA have restricted its use in consumer products. However, it remains in use for specific industrial applications, such as in fuels and plastics, where direct human exposure is limited.
Safety
The problem with azo dyes (monoazo or diazo) is photocatalytic degradation leading to oxidation and the subsequent formation of impurities such as aromatic amines, some of which have carcinogenic activity. (Chung KT, Stevens SE Jr, Cerniglia CE. The reduction of azo dyes by the intestinal microflora. Crit Rev Microbiol. 1992;18(3):175-90. doi: 10.3109/10408419209114557. )
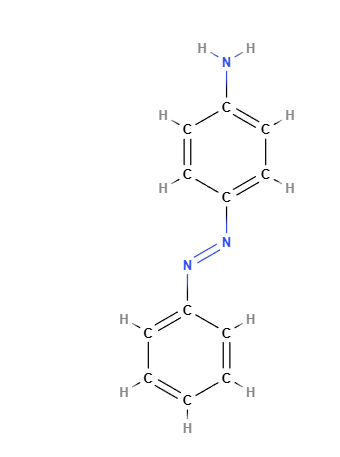 |  |
Molecular Formula C12H11N3
Molecular Weight 197.24 g/mol
CAS 60-09-3
UNII 57X2AH42T1
EC Number 200-453-6
DTXSID6024460
Synonyms:
C.I. Solvent Yellow 1
p-Aminoazobenzene
Bibliografia__________________________________________________________________________
Screening Assessment Aromatic Azo and Benzidine-based Substance GroupingCertain Azo Solvent Dyes. Environment and Climate Change Canada Health Canada May 2016
Synopsis. Pursuant to sections 68 or 74 of the Canadian Environmental Protection Act, 1999 (CEPA 1999), the Ministers of the Environment and of Health have conducted a screening assessment on 22 Azo Solvent Dyes. These substances constitute a subgroup of the Aromatic Azo and Benzidine-based Substance Grouping being assessed as part of the Groupings Initiative of Canada’s Chemicals Management Plan (CMP) based on structural similarity and applications. Substances in this grouping were identified as priorities for assessment as they met the categorization criteria under subsection 73(1) of CEPA 1999 and/or were considered as a priority based on other human health concerns. The Chemical Abstracts Service Registry Number (CAS RN)1, Domestic Substances List (DSL) name, and Colour Index (C.I) name or common name of the 22 substances are presented in the following table.....
| Evaluate |