| "Descrizione" by admin (19549 pt) | 2024-Jun-09 10:44 |
Bromocresol green is a color additive commonly used in various industrial, cosmetic, and scientific applications. It is valued for its vibrant color change properties and versatility as a pH indicator.
Chemical Composition and Structure
Bromocresol green is a synthetic dye with the chemical formula C21H14Br4O5S. It belongs to the sulfonephthalein family of dyes. The structure includes four bromine atoms, which contribute to its color-changing properties, and a complex aromatic ring system that stabilizes the molecule.
Physical Properties
Bromocresol green typically appears as a yellow crystalline powder that can range from yellow to blue-green, depending on its state and pH level. It is slightly soluble in water but more soluble in ethanol and other organic solvents. The compound is known for its stability, making it suitable for various applications where precise color change is required.
Chemical Industrial Synthesis Process
- Preparation of reagents. The main raw materials include metacresol, potassium bromide (KBr), and sulfuric acid (H₂SO₄).
- Synthesis of bromocresol. Metacresol is dissolved in concentrated sulfuric acid. Potassium bromide is slowly added to this solution with constant stirring. The reaction between metacresol and potassium bromide in the presence of sulfuric acid produces bromocresol.
- Formation of precipitate. The resulting solution is diluted with distilled water, causing the bromocresol to precipitate.
- Filtration. The suspension is filtered to separate the solid bromocresol precipitate from the aqueous solution.
- Washing. The bromocresol precipitate is washed with deionized water to remove any soluble impurities.
- Drying. The washed bromocresol is dried at controlled temperatures to remove residual moisture and obtain a dry powder.
- Grinding. The dried bromocresol is ground to obtain a fine and uniform powder. This step may involve the use of ball mills or other grinding machinery.
- Final synthesis. The bromocresol powder is treated with an alkaline solution, such as sodium hydroxide (NaOH), to form bromocresol green.
- Filtration and washing. The suspension is filtered and washed with deionized water to remove any impurities.
- Drying. The washed bromocresol green is dried at controlled temperatures to remove residual moisture and obtain a dry powder.
- Quality control. The bromocresol green undergoes rigorous quality testing to ensure it meets standards for purity, color intensity, and safety. These tests include chemical analysis and spectroscopy.
What it is used for and where
Medical
Bromocresol green is used in some medical tests and diagnostic procedures. For example, it can be used in liver function tests where the color change indicates the presence of albumin (1).
Cosmetics
Restricted cosmetic ingredient as IV/152 a Relevant Item in the Annexes of the European Cosmetics Regulation 1223/2009. Substance or ingredient reported:
- Phenol, 4,4'-(3H-2,1-benzoxathiol-3-ylidene)bis[2,6-dibromo-3-methyl-,S,S-dioxide. Maximum concentration in ready for use preparation: Rinse-off products
Cosmetics - INCI Functions
Colorant. This ingredient has the function of colouring the solution in which it is inserted in a temporary, semi-permanent or permanent manner, either alone or in the presence of the complementary components added for colouring.
Industrial Applications
pH Indicator: Bromocresol green is widely used as a pH indicator in laboratory settings. It changes color from yellow at pH levels below 3.8 to blue-green at pH levels above 5.4, making it useful for monitoring pH changes in various chemical reactions and solutions.
Aquariums and Pools: It is used to monitor the pH of water in aquariums and swimming pools. The color change provides a visual indication of water quality, helping maintain optimal conditions for aquatic life and swimmers.
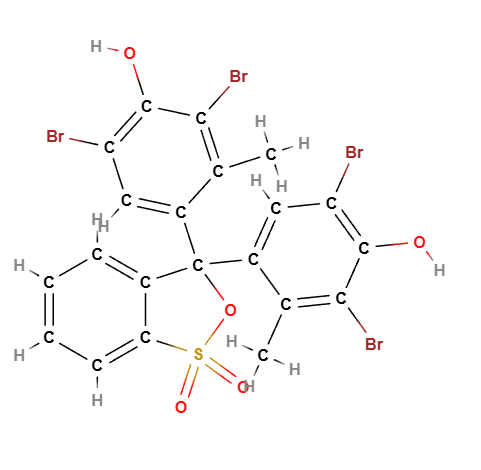 |  |
Molecular Formula C21H14Br4O5S
Molecular Weight 698.0 g/mol
CAS 76-60-8
EC number 200-972-8
UNII 8YGN0Y942M
DTXSID9044459
MFCD00005874
Synonyms:
NSC-7817
References__________________________________________________________________________
(1) Delanghe S, Biesen WV, Velde NV, Eloot S, Pletinck A, Schepers E, Glorieux G, Delanghe JR, Speeckaert MM. Binding of bromocresol green and bromocresol purple to albumin in hemodialysis patients. Clin Chem Lab Med. 2018 Feb 23;56(3):436-440. doi: 10.1515/cclm-2017-0444.
Abstyract. Background: Colorimetric albumin assays based on binding to bromocresol purple (BCP) and bromocresol green (BCG) yield different results in chronic kidney disease. Altered dye binding of carbamylated albumin has been suggested as a cause. In the present study, a detailed analysis was carried out in which uremic toxins, acute phase proteins and Kt/V, a parameter describing hemodialysis efficiency, were compared with colorimetrically assayed (BCP and BCG) serum albumin....Conclusions: In renal insufficiency, the BCP/BCG ratio of serum albumin is affected by multiple factors: next to carbamylation, uremic toxins (total PCS) and α1-acid glycoprotein also play a role.
Iwasaki T, Nakajima K, Hirowatari Y, Matsushita M. Evaluation of the two-point calibration bromocresol green method, showing reduced deviation from the bromocresol purple method in sera from patients with hypoalbuminemia. Ann Clin Biochem. 2023 Sep;60(5):320-327. doi: 10.1177/00045632231170554.
Abstract. Background: The bromocresol green (BCG) and bromocresol purple (BCP) methods are widely used for albumin measurements in routine testing, but the BCG method is known to react with globulin fractions and to have low specificity for albumin. We evaluated a calibration method using different concentrations of human serum albumin standards (two-point calibration BCG method) with the aim of reducing the effect of globulin fractions on the BCG method in patients with hypoalbuminemia.....Conclusions: Based on these results, this calibration method reduces the influence of the globulin fraction on the BCG method. In the hypoalbuminemic group, the calibration method was shown to provide results consistent with the BCP method, which is highly specific for albumin.
| Evaluate |

