Check the ingredients!
... live healthy!


| "Descrizione" by Ark90 (12417 pt) | 2023-Mar-23 14:08 |
| Evaluation | N. Experts | Evaluation | N. Experts |
|---|---|---|---|
| 1 | 6 | ||
| 2 | 7 | ||
| 3 | 8 | ||
| 4 | 9 | ||
| 5 | 10 |
Ponceau 4R or E124 is a chemical compound, one of the rarely allowed synthetic azo dyes and is frequently used in the dyeing of beverages, food, medicines, cosmetics, tobacco, textiles. It takes the number CI 16255 and is also known as Acid red 18.
It has hundreds of synonyms as it is the generic name for a family of azo dyes.
Safety. The problem with azo dyes (monoazo or diazo) is photocatalytic degradation leading to oxidation and the subsequent formation of impurities such as aromatic amines, some of which have carcinogenic activity.
It is usually obtained by a chemical synthesis process from aromatic hydrocarbons; it is stable to heat, light and acid, but fades in the presence of ascorbic acid.

Applications
bakery products, flavors and pharmaceuticals, meat, snacks, beverages, condiments, ink-jet inks, textile, paint colors etc.
Typical features of Ponceau 4R commercial dye:
| Physicval appearance | Fine red powder |
| Pure dye content | 82%-91.81% |
| Volatile matter at 135°C & chlorides and sulfates | Max 11%-15% |
| Subsidiary Dyes | 1%-0.3% |
| Ether extraction | 0.2%-0.13% |
| Non-sulfonated aromatic amines | 0.001%-<0.01% |
| Water-insoluble matter | 0.20%-0.02% |
| Chlorides and sulfates % by mass | 13%-6.02% |
| Loss on drying at 135°C, 1 hour | 5% - 3.75% |
| Pb | <2PPM |
| As | <2PPM |
| Hg | <2PPM |
Relevant studies
Ponceau 4R is used as a coloring agent in many different products, such as food, drinks, medicines, cosmetics and tobacco. However, ponceau 4R also shows carcinogenic, teratogenic and mutagenic behavior in high doses. In this work, standard Raman, theoretical Raman and surface-enhanced Raman scattering (SERS) spectra have been used to investigate ponceau 4R. More specifically, density functional theory (DFT) calculations have been used to calculate the optimized Raman spectrum of ponceau 4R at the B3LYP/6-31G(d) level. This has provided a better understanding of the optimized geometry and vibrational frequencies of this dye. In addition, the experimental spectrum of ponceau 4R has been compared with the theoretical spectrum; good agreement was obtained. Finally, it has shown that using SERS the detection limit of the ponceau 4R solution can be as low as 5 μg/mL. This has been achieved by SERS measurements of ponceau 4R on a substrate of gold nanoparticles. The SERS peaks at 1030, 1236, 1356 and 1502 cm(-1) were chosen as index for semi-quantitative analysis, showing that the SERS technique provided a useful ultrasensitive method for the detection of ponceau 4R (1).
The synthetic food dyes studied were rose bengal (RB), phroxine (PL), amaranth, erythrosine B (ET), allura red, new coccine, acid red (AR), tartrazine, sunset yellow FCF, brilliant blue FCF, and indigo carmine... Result suggests that superoxide anions, originating on dyes by light irradiation, must attack drug-metabolizing enzymes. It is possible that red cosmetics containing phloxine, erythrosine, or rose bengal react with proteins on skin under lighting and may lead to rough skin (2).
Dyes are one of the most important existing pollutants in textile industrial wastewater. These compounds are often toxic, carcinogenic, and mutagenic to living organisms, chemically and photochemically stable, and non-biodegradable. Acid red 18 is one of the azo dyes that are currently used in the textile industries (3).
Molecular Formula: C20H11N2Na3O10S3
Molecular Weight: 604.461 g/mol
UNII: Z525CBK9PG
CAS: 2611-82-7 11138-24-2 12000-58-7 161628-34-8 39470-82-1 39471-00-6 51811-48-4 247151-36-6
EC Number: 220-036-2
PubChem Substance ID 329751631
MDL number MFCD00004084
Beilstein Registry Number 4122340
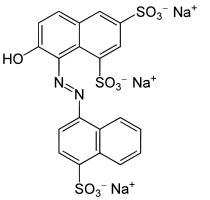
Synonyms:
References__________________________
(1) Xie Y, Li Y, Sun Y, Wang H, Qian H, Yao W. Theoretical calculation (DFT), Raman and surface-enhanced Raman scattering (SERS) study of ponceau 4R. Spectrochim Acta A Mol Biomol Spectrosc. 2012 Oct;96:600-4. doi: 10.1016/j.saa.2012.06.055.
(2) Mizutani T. Toxicity of xanthene food dyes by inhibition of human drug-metabolizing enzymes in a noncompetitive manner. J Environ Public Health. 2009;2009:953952. doi: 10.1155/2009/953952.
(3) Jorfi S, Barkhordari MJ, Ahmadi M, Jaafarzadeh N, Moustofi A, Ramavandi B. Photodegradation of Acid red 18 dye by BiOI/ZnO nanocomposite: A dataset. Data Brief. 2017 Nov 24;16:608-611. doi: 10.1016/j.dib.2017.11.068.
| Evaluate |