| "Descrizione" by Ark90 (12431 pt) | 2023-Mar-29 18:14 |
Review Consensus: 10 Rating: 10 Number of users: 1
| Evaluation | N. Experts | Evaluation | N. Experts |
|---|---|---|---|
| 1 | 6 | ||
| 2 | 7 | ||
| 3 | 8 | ||
| 4 | 9 | ||
| 5 | 10 |
E211 (Sodium Benzoate) is a chemical compound, an ingredient included in the list of European food additives as preservative.
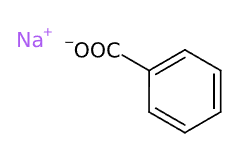
Sodium Benzoate is a chemical compound, organic sodium salt of benzoic acid.
It appears as a white, fine, odourless powder. Soluble in water, soluble in ethanol.

What it is used for and where it is used
Food
It is used as a preservative in the food industry and is labelled with the number E211 in the list of European food additives. The antibacterial effectiveness of benzoic acid and its salts, such as sodium benzoate in this case, is highly dependent on the pH of the substances in which it is added. Sodium benzoate is used intensively in soft drinks containing high-fructose corn syrup because of its power to increase acidity and taste. Because of these characteristics it is frequently found in sauces, jams, fruit juices and pickles. It is also usually found in foods containing vinegar. In minimal quantities it is found in vegetables, fruit, meat, dairy products.
Binding agent in confectionery products, as a buffer improves quality in bread.
In beer it improves diastatic quality and fermentation capacity.
Cosmetics
Used in body care products, facial care products for its anti-bacterial and anti-fungal properties.
Fragrance. It plays a very important role in the formulation of cosmetic products as it allows perfume to be enhanced, masked or added to the final product, improving its commercial viability. The consumer always expects to find a pleasant scent in a cosmetic product.
Preservative. Any product containing organic, inorganic compounds, water, needs to be preserved from microbial contamination. Preservatives act against the development of harmful microorganisms and against oxidation of the product.
Medicine
In medicine sodium benzoate is credited in this study with a potential benefit for cognitive function (1) and this other study confirmed that adjuvant therapy with sodium benzoate improved the symptomatology of patients with clozapine-resistant schizophrenia (2).
Cirrhosis and congenital errors of metabolism can lead to a serious but usually reversible neuropsychiatric complication, hepatic encephalopathy. Sodium benzoate is an inexpensive adjunct agent that can be used in addition to lactulose and rifaximin and may provide an option for selected patients with refractory hepatic encephalopathy who have not responded to standard therapies or cannot afford them (3).
High amounts of sodium benzoate may cause glycine deficiency, which may adversely affect brain neurochemistry (4).
Safety
The EFSA Panel on Food Additives considered the use of benzoic acid and its sodium and potassium salts as food additives to be of no concern with regard to genotoxicity and ruled out carcinogenic potential (5).
The most relevant studies on the subject have been selected with a summary of their contents:
Typical optimal commercial product characteristics Sodium benzoate
| Appearance | White crystalline powder |
| Boiling Point | 249.3ºC at 760 mmHg |
| Melting Point | >300 °C |
| Density | 1,44 g/cm3 |
| Flash Point | 111.4ºC |
| pH (aqueous solution) | 8 |
| Acidity and alkalinity | 0.20ml/0.1N |
| Loss on drying % | ≤15 |
| Heavy metals ppm | ≤10 ≤0.001% |
| Ammonium salts ppm | ≤25 |
| Arsenic ppm | ≤3 |
| P-toluene sulfonamide | ≤10ppm |
| O-toluene sulfonamide | ≤10ppm |
| Selenium ppm | ≤30 |
| Sulphate | ≤0.1% |
| PSA | 40.13000 |
| LogP | 0.05010 |
| Water solubility | 53.0g/100ml,25℃ |
| Ethanol solubility | 1.4g/100ml |
| 1,4-Dioxane(μg/g) | 380 max |
| Benzene(μg/g) | 2 max |
| Methylene(μg/g) | 600 max |
| Safety |  |
 | |
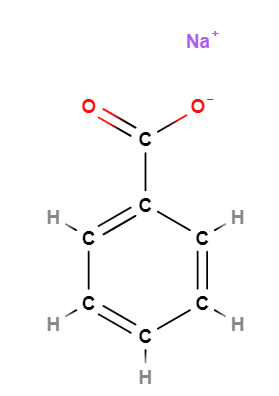 |  |
- Molecular Formula: C7H5O2Na o C7H5NaO2 o C6H5COONa o NaC6H5COO
- Molecular Weight: 144.105 g/mol
- Exact Mass 144.018723
- CAS: 532-32-1
- UNIII OJ245FE5EU
- EC Number 208-534-8
- DSSTox Substance ID DTXSID1020140
- IUPAC sodium;benzoate
- InChI=1S/C7H6O2.Na/c8-7(9)6-4-2-1-3-5-6;/h1-5H,(H,8,9);/q;+1/p-1
- InChl Key WXMKPNITSTVMEF-UHFFFAOYSA-M
- SMILES C1=CC=C(C=C1)C(=O)[O-].[Na+]
- MDL number MFCD00012463
- PubChem Substance ID 329823236
- ChEBI 113455
- ICSC 536
- RTECS DH6650000
- FEMA Number: 3025
- Beilstein 3572467
- NACRES NA.24
Synonyms:
- Benzoic acid, sodium salt
- E211
- Sobenate
- sodium;benzoate
- Antimol
- Carboxybenzene sodium salt
- Benzoic acid sodium salt
- Sodiumbenzoate
- Benzoate sodium
- BENZOTRON(R)
- Benzoate of soda
- PUROX S
- Benzoate, sodium
References___________________________________________________________________
(1) Lin CH, Yang HT, Chen PK, Wang SH, Lane HY. Precision Medicine of Sodium Benzoate for the Treatment of Behavioral and Psychological Symptoms of Dementia (BPSD). Neuropsychiatr Dis Treat. 2020 Feb 20;16:509-518. doi: 10.2147/NDT.S234371.
Lin CH, Chen PK, Wang SH, Lane HY. Sodium benzoate for the treatment of behavioral and psychological symptoms of dementia (BPSD): A randomized, double-blind, placebo-controlled, 6-week trial. J Psychopharmacol. 2019 Aug;33(8):1030-1033. doi: 10.1177/0269881119849815.
(2) Lin CH, Lin CH, Chang YC, Huang YJ, Chen PW, Yang HT, Lane HY. Sodium Benzoate, a D-Amino Acid Oxidase Inhibitor, Added to Clozapine for the Treatment of Schizophrenia: A Randomized, Double-Blind, Placebo-Controlled Trial. Biol Psychiatry. 2018 Sep 15;84(6):422-432. doi: 10.1016/j.biopsych.2017.12.006.
(3) Misel ML, Gish RG, Patton H, Mendler M. Sodium benzoate for treatment of hepatic encephalopathy. Gastroenterol Hepatol (N Y). 2013 Apr;9(4):219-27.
(4) Piper JD, Piper PW. Benzoate and Sorbate Salts: A Systematic Review of the Potential Hazards of These Invaluable Preservatives and the Expanding Spectrum of Clinical Uses for Sodium Benzoate. Compr Rev Food Sci Food Saf. 2017 Sep;16(5):868-880. doi: 10.1111/1541-4337.12284.
(5) EFSA Panel on Food Additives and Nutrient Sources (ANS). (2016). Scientific Opinion on the re‐evaluation of benzoic acid (E 210), sodium benzoate (E 211), potassium benzoate (E 212) and calcium benzoate (E 213) as food additives. EFSA Journal, 14(3), 4433.
Abstract. The EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS) was asked to deliver a scientific opinion re-evaluating benzoic acid (E 210), sodium benzoate (E 211), potassium benzoate (E 212) and calcium benzoate (E 213) when used as food additives. Benzoic acid and its sodium and potassium salts are rapidly absorbed after oral administration. The Panel considered that the absorption, distribution, metabolism and excretion of calcium benzoate will be similar to sodium or potassium salt and, therefore, read-across between the salts was possible. The results of short-term and subchronic studies on benzoic acid and its salts indicate that their toxicity is low. The Panel considered that the use of benzoic acid and its sodium and potassium salts as food additives does not raise a concern with respect to genotoxicity and, based on read-across, also considered that this conclusion is applicable for calcium benzoate. Moreover, the Panel noted that the available data did not indicate any carcinogenic potential. A four-generation reproductive toxicity study with benzoic acid in the diet in rats was considered by the Panel as the pivotal study and a no observed adverse effect level of 500 mg benzoic acid/kg body weight (bw) per day, the highest dose tested, was identified. From the aforementioned studies, the Panel derived an acceptable daily intake (ADI) of 5 mg/kg bw per day (expressed as benzoic acid) using an uncertainty factor of 100. Taking into account food categories for which direct addition of benzoic acid-benzoates is authorised, the group ADI was exceeded in the brand-loyal scenario in particular for toddlers and children consuming on a regular basis flavoured drinks. Considering additional exposure due to carry-over, the intake could be increased by up to two to three fold for all high-level consumers compared to the previous scenario with only direct addition to food. This results in exceedance of the group ADI in toddlers and children for the non-brand-loyal scenario. The main food categories contributing to this exceedance were unprocessed fruits and vegetables and flavoured drinks.
| Evaluate |

