Check the ingredients!
... live healthy!


| "Descrizione" by A_Partyns (12876 pt) | 2023-Jul-09 17:58 |
| Evaluation | N. Experts | Evaluation | N. Experts |
|---|---|---|---|
| 1 | 6 | ||
| 2 | 7 | ||
| 3 | 8 | ||
| 4 | 9 | ||
| 5 | 10 |
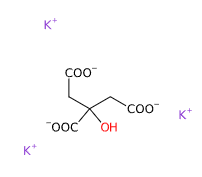
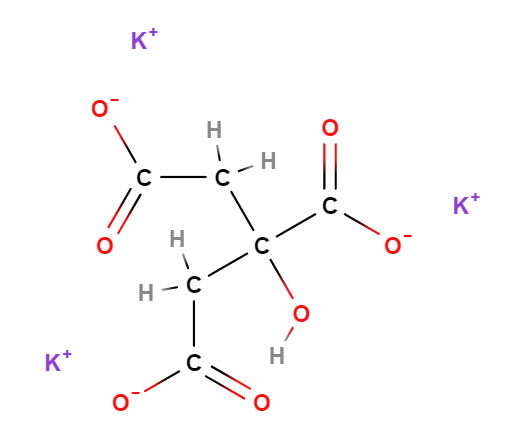
E332 (Potassium citrate) is a chemical compound, inorganic salt of citric acid (α and β-hydroxytricarboxylic acid).
The name 'potassium citrate' can be broken down as follows:
Potassium citrate synthesis can be achieved by neutralising citric acid with potassium hydroxide in different steps:
The industrial production of citric acid takes place by mycological fermentation of raw sugar from Aspergillus niger and subsequent crystallisation with alkaline solutions.
It appears as an odourless white crystalline powder with a slightly saline taste. Slightly hygroscopic, easily soluble in water, slow to dissolve in glycerine, insoluble in alcohol and ethanol.

What it is used for and where
Medical
In addition to its main function as a preservative, Potassium Citrate is also a decent antimicrobial, although inferior to Potassium Acetate (1). Potassium citrate is used to improve bone mineral density and to prevent metabolic acidosis in kidney disease (2) and has enhanced the beneficial effects of calcium and vitamin D in osteopenic women with a documented potassium and citrate deficiency and a metabolic profile compatible with low-grade acidosis (3). It has been shown that potassium citrate can increase urine pH values and dissolve kidney stones (4) and that alkaline therapy increases bone mineral density and corrects abnormal bone cell function.
Some improvement has been achieved in the treatment of dehydration by potassium citrate supplementation.
It has been observed that potassium citrate, with its alkanising action, can alleviate the discomfort of cystitis caused by lower urinary tract infections (5).
Indicated in the treatment of potassium deficiency.
Studies have shown its efficacy in strengthening bones and thus being less prone to fractures and osteoporosis.
Dentistry
The inclusion of potassium citrate in toothpastes reduced tooth sensitivity after 3 weeks (6) and has an antibacterial function.
Food
It is labelled E332 on the European food additives list as an acidity regulator, but also has antiseptic properties. Emulsifier in cheese.
Cosmetics
Chelating and buffering agent in cosmetic products with preservative properties.
Typical commercial product characteristics Potassium Citrate
| Appearance | White powder |
| pH | 7.5-9.0 |
| Boiling Point | 309.6ºC at 760 mmHg |
| Flash Point | 155.2ºC |
| Density | 1.187 |
| PSA | 140.62000 |
| Chlorides | 0.005% |
| Sulphate | 0.015% |
| Oxalate | 0.03% |
| Heavy Metal | ≤10ppm |
| As | ≤2ppm |
| Loss on drying | 3.0-6.0 (180°C, 4h) |
| Fe | ≤5mg/kg |
| Pb | ≤2ppm |
| Ca | ≤0.02% |
| Hg | ≤0.1ppm |
| Cd | ≤1ppm |
| Shelf Life | 2 Years |
 |  |
 |  |
Synonyms:
References________________________________________________________________________
(1) Liato V, Labrie S, Aïder M. Electro-activation of potassium acetate, potassium citrate and calcium lactate: impact on solution acidity, Redox potential, vibrational properties of Raman spectra and antibacterial activity on E. coli O157:H7 at ambient temperature. Springerplus. 2016 Oct 10;5(1):1760. doi: 10.1186/s40064-016-3453-1.
(2) Domrongkitchaiporn S, Pongskul C, Sirikulchayanonta V, Stitchantrakul W, Leeprasert V, Ongphiphadhanakul B, Radinahamed P, Rajatanavin R. Bone histology and bone mineral density after correction of acidosis in distal renal tubular acidosis. Kidney Int. 2002 Dec;62(6):2160-6. doi: 10.1046/j.1523-1755.2002.00656.x.
(3) Granchi D, Caudarella R, Ripamonti C, Spinnato P, Bazzocchi A, Massa A, Baldini N. Potassium Citrate Supplementation Decreases the Biochemical Markers of Bone Loss in a Group of Osteopenic Women: The Results of a Randomized, Double-Blind, Placebo-Controlled Pilot Study. Nutrients. 2018 Sep 12;10(9):1293. doi: 10.3390/nu10091293.
(4) Barbera M, Tsirgiotis A, Barbera M, Paola Q. The importance of potassium citrate and potassium bicarbonate in the treatment of uric acid renal stones. Arch Ital Urol Androl. 2016 Dec 30;88(4):341-342. doi: 10.4081/aiua.2016.4.341.
(5) Elizabeth JE, Carter NJ. Potassium citrate mixture: soothing but not harmless? Br Med J (Clin Res Ed). 1987 Oct 17;295(6604):993. doi: 10.1136/bmj.295.6604.993.
(6) Chesters R, Kaufman HW, Wolff MS, Huntington E, Kleinberg I. Use of multiple sensitivity measurements and logit statistical analysis to assess the effectiveness of a potassium-citrate-containing dentifrice in reducing dentinal hypersensitivity. J Clin Periodontol. 1992 Apr;19(4):256-61. doi: 10.1111/j.1600-051x.1992.tb00463.x.
| Evaluate |