| "Descrizione" by MGannelly (1342 pt) | 2024-Oct-14 16:07 |
Review Consensus: 9 Rating: 9 Number of users: 1
| Evaluation | N. Experts | Evaluation | N. Experts |
|---|---|---|---|
| 1 | 6 | ||
| 2 | 7 | ||
| 3 | 8 | ||
| 4 | 9 | ||
| 5 | 10 |
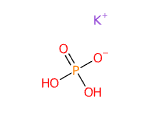
Potassium Phosphate, identified by the code E340 as a food additive, is an inorganic chemical compound composed of potassium and phosphoric acid. It is primarily used as an acidity regulator, emulsifier, and stabilizer in food products. It is available in various forms, including monopotassium, dipotassium, and tripotassium phosphate, each with specific chemical properties and applications. Potassium phosphate is commonly found in processed foods such as beverages, powdered products, and baked goods, where it plays an important role in maintaining pH balance and stabilizing emulsions.
Chemical Composition and Structure
Potassium phosphate exists in different forms:
- Monopotassium phosphate (KH₂PO₄)
- Dipotassium phosphate (K₂HPO₄)
- Tripotassium phosphate (K₃PO₄)
These variations result from the number of hydrogen atoms replaced by potassium atoms in the phosphoric acid structure. Their chemical formulas reflect their ability to neutralize acids, making these variants useful in different food and industrial contexts.
Physical Properties
Potassium phosphate is a white crystalline solid, odorless, and water-soluble. It has pH-regulating properties, making it useful for balancing acidity in food products. In both liquid and powder form, it is easily mixable with other food ingredients, facilitating the stabilization of formulations.

Production Process
Potassium phosphate is typically produced by reacting phosphoric acid with potassium hydroxide or potassium carbonate. This chemical reaction yields the different forms of potassium phosphate, which can be purified and crystallized for use in food or other applications.
Selezione delle Materie Prime: Gli ingredienti principali per la produzione del fosfato di potassio includono acido fosforico e idrossido di potassio. Questi composti chimici sono facilmente reperibili e vengono scelti per la loro purezza e qualità.
Reazione di Neutalizzazione: L'acido fosforico viene miscelato con idrossido di potassio in un reattore chimico. Questa reazione avviene in condizioni controllate di temperatura e pH, dove l'acido e la base si neutralizzano per formare fosfato di potassio.
Cristallizzazione: Dopo la reazione, la soluzione contenente fosfato di potassio viene raffreddata per consentire la cristallizzazione del prodotto. I cristalli di fosfato di potassio vengono poi raccolti.
Purificazione: I cristalli di fosfato di potassio ottenuti vengono purificati per rimuovere eventuali impurità e residui. Questo può includere processi di lavaggio e filtrazione per garantire un prodotto di alta qualità.
Controllo Qualità e Confezionamento: Infine, il fosfato di potassio viene sottoposto a controlli di qualità per verificarne la purezza e le proprietà funzionali. Dopo l'analisi, viene confezionato per la distribuzione e l'uso in prodotti alimentari e cosmetici.
Food
In the food industry, it is used as an acidity regulator and to prevent the formation of lumps in baked goods.
An ingredient included in the list of European food additives as E340 acidity regulator and chelating agent with the following specifications :
- E340 (i) Monopotassium phosphate CAS 7778-77-0
- E340 (ii) Dipotassium phosphate CAS 7758-11-4
- E340 (iii) Tripotassium phosphate CAS 7778-53-2
Medical
Potassium phosphate is widely used to control hypophosphatemia, i.e. phosphate deficiency in the blood, and to prevent the formation of kidney stones.
Cosmetics
Antibacterial and to prevent the formation of lumps.
Buffering agent. It is an iingredient that can bring an alkaline or acid solution to a certain pH level and prevent it from changing, in practice a pH stabiliser that can effectively resist instability and pH change.
Other uses
As an industrial product, it is made from phosphoric acid and has numerous applications in various sectors:
- agricultural fertilisers
- forestry
- fishing
Safety
The Panel on Food Additives and Flavourings added to Food (FAF) provided a scientific opinion re-evaluating the safety of phosphates (E 338–341, E 343, E 450–452) as food additives. The Panel considered that (1). The Panel considered that phosphates have low acute oral toxicity and there is no concern regarding genotoxicity and carcinogenicity. The Panel calculated a group Acceptable Daily Intake (ADI) for phosphates expressed as phosphorus of 40 mg/kg body weight (bw) per day and concluded that this ADI is protective for the human population (1).
 |  |
 |
- Molecular Formula: K3O4P K3PO4
- Molecular Weight: 212.265 g/mol
- UNII: 16D59922JU
- CAS: 7778-53-2 14887-42-4 16068-46-5 44042-47-9 1017687-06-7 1269628-80-9 1608125-41-2 2139271-06-8
- EC Number: 231-907-1 238-961-5 240-213-8 920-994-8
- PubChem Substance ID 57654540
- MDL number MFCD00036295
Synonyms:
- Tripotassium phosphate
- E340
- tri-Potassium orthophosphate
- Potassium phosphate, anhydrous
- Tripotassium phosphate,anhydrous
- Potassium phosphate tribasic anhydrous
- phosphoric acid potassium salt
- Phosphate buffer
- potassiumphosphate
- potasium phosphate
- tri potassium phosphate
- tri-potassium phosphate
- Tripotassium phosphate|
- tris-potassium phosphate
- potassium phophate
- potassium phoshate
- potassium-phosphate
- Tripotasium phosphate
- tripotassium,phosphate
- tripotassiurn phosphate
- Potassium phosphate tribasic
- Tripotassium orthophosphate
- Phosphoric acid, tripotassium salt
- Potassium orthophosphate
- Caswell No. 700A
- Phosphoric acid, potassium salt
- Potassium phosphate, tribasic
References_______________________________________________________________________
(1) EFSA Panel on Food Additives and Flavourings (FAF); Younes M, Aquilina G, Castle L, Engel KH, Fowler P, Frutos Fernandez MJ, Fürst P, Gürtler R, Husøy T, Mennes W, Moldeus P, Oskarsson A, Shah R, Waalkens-Berendsen I, Wölfle D, Aggett P, Cupisti A, Fortes C, Kuhnle G, Lillegaard IT, Scotter M, Giarola A, Rincon A, Tard A, Gundert-Remy U. Re-evaluation of phosphoric acid-phosphates - di-, tri- and polyphosphates (E 338-341, E 343, E 450-452) as food additives and the safety of proposed extension of use. EFSA J. 2019 Jun 12;17(6):e05674. doi: 10.2903/j.efsa.2019.5674.
| Evaluate |

