Check the ingredients!
... live healthy!


| "Descrizione" by Frank123 (12008 pt) | 2023-Apr-13 17:17 |
| Evaluation | N. Experts | Evaluation | N. Experts |
|---|---|---|---|
| 1 | 6 | ||
| 2 | 7 | ||
| 3 | 8 | ||
| 4 | 9 | ||
| 5 | 10 |
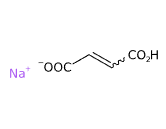
E365 (Sodium fumarate) è un composto chimico, sale di sodio dell'acido fumarico ottenuto sinteticamente dalla reazione dell'acido fumarico con idrossido di sodio a pH 6,2.
Si presenta in forma di polvere biancastra altamente solubile in acqua.

What it is used for and where
Food
Ingredient listed in the European food additives list as E365, acidity regulator.
Cosmetics
Buffering agent. It is an iingredient that can bring an alkaline or acid solution to a certain pH level and prevent it from changing, in practice a pH stabiliser that can effectively resist instability and pH change.
Animal nutrition
Sodium fumarate is usually mixed with nutritional additives fed to newborn pigs after the cessation of breastfeeding to prevent weight loss due to excessive bacterial growth. It is also an effective agent to prevent a drop in ruminal pH by raising the pH.
Other uses
Sodium fumarate is one of the initial compounds in the synthesis of ferrous fumarate.
 |  |
 |  |
Synonyms:
Studies
Asanuma, N., Iwamoto, M. and Hino, T., 1999. Effect of the addition of fumarate on methane production by ruminal microorganisms in vitro. Journal of Dairy Science, 82(4), pp.780-787.
Mrowietz, U. (2003). Clinical Use and Pharmacological Profile of Fumaric Acid Esters. Textbook of Psoriasis, 311-326.
Skuban, S., Dzomié, T., Kapor, A., Cvejic, Z. and Rakic, S., 2012. Dielectric and structural properties of iron-and sodium-fumarates. Journal of Research in Physics, 36(1), p.21.
Creveling, C. R., & Daly, J. W. (1971). Assay of enzymes of catecholamine biosynthesis and metabolism. Methods of Biochemical Analysis (suppl. vol.), ed. by D. Glick, 153-182.
Zhang, J., Zhao, J., Wang, J., Tabish, M. and Zhang, J., 2023. Enhancing the corrosion resistance of waterborne epoxy coating by fumarate intercalated LDHs prepared by high gravity technology. Progress in Organic Coatings, 174, p.107271.
Zhang, K. (2012). Fumaric acid fermentation by Rhizopus oryzae with integrated separation technologies. The Ohio State University.
| Evaluate |