Check the ingredients!
... live healthy!


| "Descrizione" by Frank123 (12008 pt) | 2024-May-31 15:11 |
| Evaluation | N. Experts | Evaluation | N. Experts |
|---|---|---|---|
| 1 | 6 | ||
| 2 | 7 | ||
| 3 | 8 | ||
| 4 | 9 | ||
| 5 | 10 |
E516 (Calcium sulfate) is a chemical compound, sulfuric acid calcium salt.
Calcium sulfate is chemically classified as an inorganic colorant. This pigment is widely used in various industrial and cosmetic applications due to its excellent physical and chemical properties, including high stability, non-reactivity, and versatility.
Chemical Composition and Structure
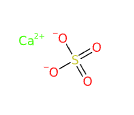
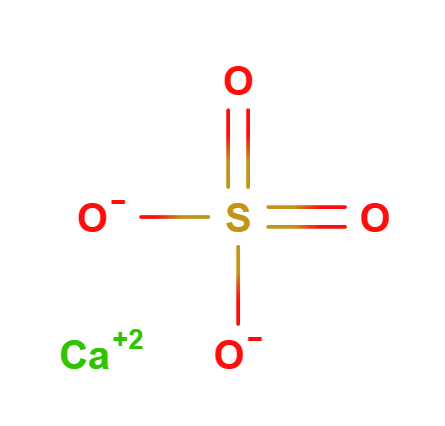
Calcium sulfate is an inorganic compound with the chemical formula CaSO4. It consists of calcium (Ca), sulfur (S), and oxygen (O), forming calcium sulfate. This compound is known for its insolubility in water and its high stability, making it useful as a pigment and filler in numerous applications.
Physical Properties
This pigment typically appears as a fine white powder. It has a high density and excellent covering power. Calcium sulfate is known for its chemical stability, resistance to light and heat, and non-reactive nature, making it safe for a wide range of applications.
Chemical Industrial Synthesis Process
It comes in the form of white or yellowish powder.

What it is used for and where
Food
Ingredient included in the list of European food additives such as E516 with the function of an acidity regulating agent.
Cosmetics
Restricted cosmetic ingredient as IV/125 a Relevant Item in the Annexes of the European Cosmetics Regulation 1223/2009.
Cosmetics - INCI Functions
Calcium sulfate is used in cosmetic products such as foundations, powders, and eyeshadows for its high opacity and ability to improve the texture of products. It acts as a bulking agent and helps in achieving a smooth and matte finish.
Industrial Applications
Paints and Coatings: In this sector, calcium sulfate is used as an extender to improve the coverage and durability of paints. Its stability makes it suitable for exterior and decorative applications.
Plastics and Polymers: Calcium sulfate is employed in the production of plastics and polymers to enhance the strength and stability of finished products. It provides uniform color and resists degradation from UV light and heat.
Paper: Calcium sulfate is used as a filler in paper production, improving the brightness, opacity, and overall quality of the paper product.
Construction: In the construction industry, it is used in the formulation of plasters, cements, and drywall due to its excellent binding properties and stability.
Safety
The EFSA Scientific Panel on Food Additives has provided a scientific opinion reviewing the safety of sulphuric acid (E513) salts and their sodium (E514), potassium (E515), calcium (E516) and ammonium (E517) salts when used as food additives. The Scientific Panel noted that exposure to sulphates on average and at 95 percentile in the non-brand scenario, as well as in other scenarios, is far lower than the dose of 300 mg/kg which induced a laxative effect in humans. Based on the available toxicological database, the Scientific Panel concluded that exposure to sulphuric acid (E513), sodium sulfate (E514), potassium sulfate (E515), calcium sulfate (E516) and ammonium sulfate (E517) does not raise safety concerns for declared uses and use levels and no numerical acceptable daily intake (ADI) is required (1).
Calcium sulfate is generally considered safe for use in consumer products when handled following proper safety procedures. It is non-toxic and environmentally stable. However, as with all powdered materials, precautions should be taken to avoid inhalation and minimize direct contact with skin and eyes.
 |  |
 |  |
Formula molecolare CaSO4 CaO4S
Peso molecolare 136.14
CAS 7778-18-9
UNII E934B3V59H
EC Number 231-900-3
References_____________________________________________________________________
(1) EFSA Panel on Food Additives and Flavourings (FAF), Younes, M., Aquilina, G., Castle, L., Engel, K.H., Fowler, P., Fürst, P., Gürtler, R., Gundert‐Remy, U., Husøy, T. and Mennes, W., 2019. Re‐evaluation of sulphuric acid and its sodium, potassium, calcium and ammonium salts (E 513, 514 (i), 514 (ii), 515 (i), 515 (ii), 516 and 517) as food additive. EFSA Journal, 17(10), p.e05868.
| Evaluate |