Check the ingredients!
... live healthy!


| "Descrizione" by Whiz35 (11828 pt) | 2024-Jan-14 19:07 |
| Evaluation | N. Experts | Evaluation | N. Experts |
|---|---|---|---|
| 1 | 6 | ||
| 2 | 7 | ||
| 3 | 8 | ||
| 4 | 9 | ||
| 5 | 10 |
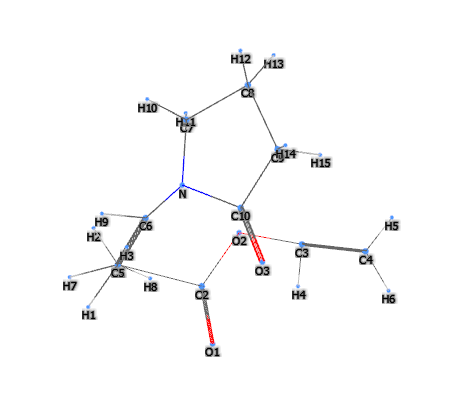
E1208 (Polyvinylpyrrolidone vinyl acetate copolymer or PVP/VA Copolymer) is a chemical compound, copolymer or copolymers that can be random or linear and are produced synthetically by the process of 'radical polymerisation' whereby a conversion of double bonds present in the monomer unit takes place, transforming them into saturated ones. This is the product of conventional radical copolymerisation of N-vinyl-2-pyrrolidone and vinyl acetate in a propan-2-ol solution with initiators.
The ratios of vinyl acetate to vinylpyrrolidone. can vary between 30/70 and 70/30. Polymers with 30%, 50%, 60% and 70% vinylpyrrolidone content are produced in isopropanol and ethanol. Polyvinylpyrrolidone vinylacetate copolymers with a vinylpyrrolidone content of 60 and 70 per cent are either 50 per cent aqueous solutions or solids.
The name describes the structure of the molecule:
Origin. Chemically synthesized by combining polyvinylpyrrolidone and vinyl acetate.
Industrial Production Process
It appears as a white to yellowish-white powder or flakes, with an average particle size of 50-130 μm freely soluble in water, ethanol, ethylene chloride and ether, or as a transparent liquid soluble in water, ethanol, isopropanol.

What it is used for and where
Binding/coating agent
Food
In 2009, an application was submitted to the European Commission for authorisation of polyvinylpyrrolidone vinylacetate in solid food supplements: 'Food supplements in solid form, including capsules, tablets and the like, except chewing tablets. Polyvinylpyrrolidone vinylacetate copolymer improves the strength and adhesion of the coating film and increases the rate of application.
Authorization was allowed in 2018. Full text:
The additive is authorised to be used in the following category(ies):
17.1Food supplements supplied in a solid form (COMMISSION REGULATION (EU) 2018/1497
of 8 October 2018
amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council as regards food category 17 and the use of food additives in food supplements
(Text with EEA relevance), applicable from 29/10/2018)
Individual restriction(s) / exception(s)ML = 100000 mg/kg, except food supplements in chewable form
It is labelled with the number E1208 in the European food additives list as a coating agent.
Medical and Pharmaceuticals
Polyvinylpyrrolidone vinylacetate copolymer is usually associated with low concentrations of active pharmaceutical ingredients and the mechanism of drug release is controlled by the polymer (1).
Safety
According to the opinion of Commission Regulation (EU) No 264/2014 of 14 March 2014, polyvinylpyrrolidone vinyl acetate copolymer as a binding/coating agent in solid food supplements is not expected to present safety concerns (2).
The most relevant studies on this ingredient have been selected with a summary of their contents:
Typical commercial product characteristics PVP/VA Copolymer
| Appearance | White powder |
| Nitrogen content | 7,0-8,0 % |
| pH | 3,0 – 7,0 |
| Vinyl acetate | 42,0 % |
| Free vinyl acetate | 5 mg/kg |
| Total ash | 0,1 % |
| Free N-vinylpyrrolidone | 5 mg/kg |
| Hydrazine | 0,8 mg/kg |
| Peroxide | 400 mg/kg |
| Propan-2-ol | 150 mg/kg |
| As | 3 mg/kg |
| Pb | 2 mg/kg |
| Mercury | 1 mg/kg |
| Cadmium | 1 mg/kg |
 |  |
 |  |
Synonyms
References________________________________________________________________________
(1) Ritters L, Tian Y, Reichl S. Spray-Dried Paracetamol/Polyvinylpyrrolidone Amorphous Solid Dispersions: Part I-Stability of Powders and Tablets. Pharmaceutics. 2021 Nov 16;13(11):1938. doi: 10.3390/pharmaceutics13111938.
Abstract. The formulation of active pharmaceutical ingredients (APIs) in amorphous solid dispersions (ASDs) is a promising approach to improve the bioavailability of poorly soluble compounds. However, problems often arise in the production of tablets from ASDs regarding the compressibility and recrystallization of the API. In the present study, the preparation of spray-dried ASDs of paracetamol (PCM) and four different types of polyvinylpyrrolidone (PVP) and their further processing into tablets were investigated. The influence of PVP type on the glass transition temperature (Tg) and the physical stability of ASD powders were characterized by differential scanning calorimetry (DSC) and powder X-ray diffraction (XRD). ASD powders with 10 to 30% PCM were stable for at least 48 weeks. PCM contents of 40 to 50% led to recrystallization of the amorphous PCM within a few days or weeks. ASD with PVP/vinyl acetate (VA) copolymer (PVP/VA) was the most unstable and tended to recrystallize in PCM polymorphic form II. This formulation was therefore used for tablet studies. The influence of compression force on recrystallization, crushing strength, and drug release was investigated. Even high compression forces did not affect the stability of the ASD. However, the ASD tablets led to slow release of the API.
(2) L_2014076IT.01002201.xml (europa.eu)
| Evaluate |