| "Descrizione" by Whiz35 (11840 pt) | 2023-Apr-29 20:15 |
Review Consensus: 8 Rating: 8 Number of users: 1
| Evaluation | N. Experts | Evaluation | N. Experts |
|---|---|---|---|
| 1 | 6 | ||
| 2 | 7 | ||
| 3 | 8 | ||
| 4 | 9 | ||
| 5 | 10 |
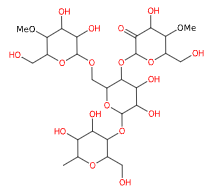
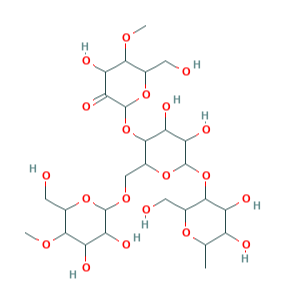
E1404 (Oxidised starch) is a modified starch obtained by acid, enzymatic, alkaline or bleaching treatment with sodium hypochlorite and the associated production of carboxyl groups.
It appears as a white powder or white granules.

What it is used for and where
Food
Ingredient included in the list of European food additives as E1404 with the function of thickening and coating agent. It is used as a substitute for gum arabic in flavour encapsulation (1).
Safety
The EFSA Panel on Food Additives and Nutrient Sources Added to Food considers that there is no safety concern for the use of modified starches as food additives at the uses and use levels declared for the general population and that a numerical ADI is not necessary (2).
 |  |
 |
- Molecular Formula C27H46O20
- Molecular Weight 690.6
- CAS 65996-62-5
- UNII
- EC Number 613-862-3.
References_____________________________________________________________________
(1) Chattopadhyaya, S., Singhal, R.S. and Kulkarni, P.R., 1998. Oxidised starch as gum arabic substitute for encapsulation of flavours. Carbohydrate Polymers, 37(2), pp.143-144.
Abstract. Oxidised starches prepared from corn and waxy amaranth starch under conditions optimised for development for film forming ability were compared with gum arabic and a known substitute of gum arabic for encapsulation of a model flavour compound, vanillin. Percentage vanillin encapsulated using gum arabic, amiogum 688 (a known gum arabic substitute), oxidised corn starch and oxidised amaranth starch differed marginally and were found to be 57.84%, 58.61%, 60.89% and 58.61% respectively of the recoverable vanillin. Results obtained suggest the possibility of using oxidised starch as a substitute for gum arabic in encapsulated flavours with advantages such as freedom from hygroscopicity and similar encapsulation efficiency.
(2) EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS), Mortensen, A., Aguilar, F., Crebelli, R., Di Domenico, A., Dusemund, B., Frutos, M.J., Galtier, P., Gott, D., Gundert‐Remy, U. and Lambré, C., 2017. Re‐evaluation of oxidised starch (E 1404), monostarch phosphate (E 1410), distarch phosphate (E 1412), phosphated distarch phosphate (E 1413), acetylated distarch phosphate (E 1414), acetylated starch (E 1420), acetylated distarch adipate (E 1422), hydroxypropyl starch (E 1440), hydroxypropyl distarch phosphate (E 1442), starch sodium octenyl succinate (E 1450), acetylated oxidised starch (E 1451) and starch aluminium octenyl succinate (E 1452) as food additives. EFSA Journal, 15(10), p.e04911.
Abstract. Following a request from the European Commission, the EFSA Panel on Food Additives and Nutrient sources added to Food (ANS) was asked to deliver a scientific opinion on the re-evaluation of 12 modified starches (E 1404, E 1410, E 1412, E 1413, E 1414, E 1420, E 1422, E 1440, E 1442, E 1450, E 1451 and E 1452) authorised as food additives in the EU in accordance with Regulation (EC) No 1333/2008 and previously evaluated by JECFA and the SCF. Both committees allocated an acceptable daily intake (ADI) ‘not specified’. In humans, modified starches are not absorbed intact but significantly hydrolysed by intestinal enzymes and then fermented by the intestinal microbiota. Using the read-across approach, the Panel considered that adequate data on short- and long-term toxicity and carcinogenicity, and reproductive toxicity are available. Based on in silico analyses, modified starches are considered not to be of genotoxic concern. No treatment-related effects relevant for human risk assessment were observed in rats fed very high levels of modified starches (up to 31,000 mg/kg body weight (bw) per day). Modified starches (e.g. E 1450) were well tolerated in humans up to a single dose of 25,000 mg/person. Following the conceptual framework for the risk assessment of certain food additives, the Panel concluded that there is no safety concern for the use of modified starches as food additives at the reported uses and use levels for the general population and that there is no need for a numerical ADI. The combined exposure to E 1404–E 1451 at the 95th percentile of the refined (brand-loyal) exposure assessment scenario for the general population was up to 3,053 mg/kg bw per day. Exposure to E 1452 for food supplement consumers only at the 95th percentile was up to 22.1 mg/kg bw per day.
| Evaluate |

