| "Descrizione" by Street82 (2970 pt) | 2023-May-18 19:08 |
Review Consensus: 10 Rating: 10 Number of users: 1
| Evaluation | N. Experts | Evaluation | N. Experts |
|---|---|---|---|
| 1 | 6 | ||
| 2 | 7 | ||
| 3 | 8 | ||
| 4 | 9 | ||
| 5 | 10 |
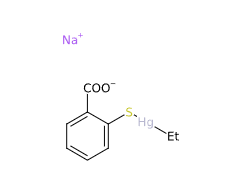
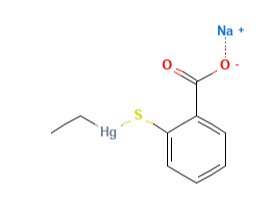
Sodium ethylmercurythiosalicylate (Thimerosal or Tiomersal) or is an organomercury compound that comprises 49.55% (w/w) mercury in its structure and degrades to produce thiosalicylic acid, dithiobenzoic acid and ethylmercury (1).
It appears in the form of a beige powder incompatible with iodine, heavy metal salts, strong acids, strong bases, strong oxidizing agents.

What it is used for and where
Medical
Due to its bacteriostatic and fungistatic properties, Tiomersal is used as a preservative in vaccines against hepatitis B, influenza, diphtheria, tetanus, pertussis, meningococcal disease and Haemophilus influenzae type B. The typical concentration of Tiomersal in vaccines is 0.01%.
Safety
The WHO considers small doses of Tiomersal to be safe even with multiple and repetitive exposures to vaccines administered during childhood or to pregnant women.
On its safety, some studies considered that current knowledge on this subject (2013) is still incomplete and further studies are needed (2), while the most recent scientific literature (2022) have highlighted the toxicity of mercury that would lead to structural and functional changes in macromolecules such as haemoglobin in erythrocytes (3).
In this 2004 study, Tiomersal or Thimerosal is indicated as a true ophthalmic allergen in periorbital allergic contact dermatitis (4).
It may be useful to perform a preventive patch-test before taking this chemical to check that no allergic problems arise.
Cosmetics
It is a restricted ingredient V/16 a Relevant Item in the Annexes of the European Cosmetics Regulation 1223/2009. Maximum concentration in ready for use preparation 0.007% (Hg).If mixed with other mercurial compounds authorized by this Regulation, the maximum concentration of Mercury remains fixed at 0.007%
Other uses
Paints, industrial catalyst.
The most relevant studies on this chemical compound have been selected with a summary of their contents:
Typical commercial product characteristics Thiomersal
| Appearance | Beige powder |
| pH | 6.0 - 8.0 |
| Boiling Point | 298.6ºC at 760mmHg |
| Melting Point | 234-237 °C |
| Flash Point | 250 °C |
| PSA | 65.43000 |
| LogP | 1.57790 |
| Free mercury ions | ≤ 0.70 % |
| Loss on drying | ≤ 0.5 % |
| Diethyl ether | ≤ 5000 ppm |
| Ethanol | ≤ 5000 ppm |
| Safety |  |
 |  |
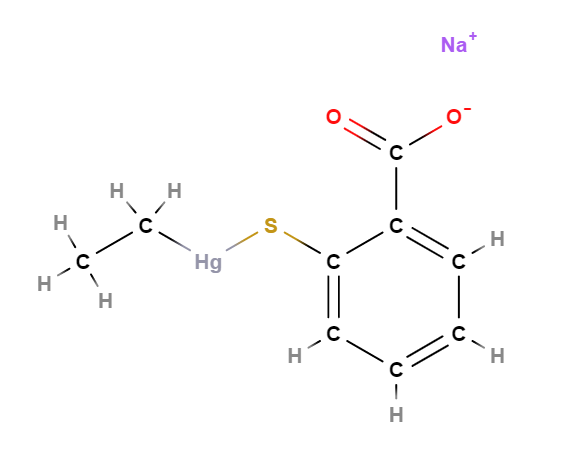 |
- Molecular Formula C9H9HgO2S.Na C9H9HgNaO2S
- Linear Formula 2-(C2H5HgS)C6H4CO2Na
- Molecular Weight 404.82
- Exact Mass 405.992706
- CAS 54-64-8
- UNII 2225PI3MOV
- EC Number 200-210-4
- DSSTox Substance ID DTXSID3025540
- IUPAC sodium;(2-carboxylatophenyl)sulfanyl-ethylmercury
- InChI=1S/C7H6O2S.C2H5.Hg.Na/c8-7(9)5-3-1-2-4-6(5)10;1-2;;/h1-4,10H,(H,8,9);1H2,2H3;;/q;;2*+1/p-2
- InChl Key RTKIYNMVFMVABJ-UHFFFAOYSA-L
- SMILES CC[Hg]SC1=CC=CC=C1C(=O)[O-].[Na+]
- MDL number MFCD00013062
- PubChem Substance ID 24900219
- ChEBI 9546
- NCI C47751
- RXCUI 10472
- Beilstein 8169555
- RTECS OV8400000
- UN 2025
Synonyms
- Sodium ethylmercurythiosalicylate
- 2-(Ethylmercuriomercapto)benzoic acid sodium salt
- Mercury-([o-carboxyphenyl]thio)ethyl sodium salt
- Thimerosal
- Thiomersal
References______________________________________________________________________
(1) Pedrozo-Penafiel MJ, Miranda-Andrades JR, Gutierrez-Beleño LM, Larrudé DG, Aucelio RQ. Indirect voltammetric determination of thiomersal in influenza vaccine using photo-degradation and graphene quantum dots modified glassy carbon electrode. Talanta. 2020 Aug 1;215:120938. doi: 10.1016/j.talanta.2020.120938.
(2) Dórea JG, Farina M, Rocha JB. Toxicity of ethylmercury (and Thimerosal): a comparison with methylmercury. J Appl Toxicol. 2013 Aug;33(8):700-11. doi: 10.1002/jat.2855.
(3) Sales MVS, da Silva Filho RC, Silva MM, Rocha JL, Freire RO, de L Tanabe EL, Silva ECO, Fonseca EJS, Figueiredo IM, Rocha U, Santos JCC, Leite ACR. Consequences of thimerosal on human erythrocyte hemoglobin: Assessing functional and structural protein changes induced by an organic mercury compound. J Trace Elem Med Biol. 2022 Jan 10;71:126928. doi: 10.1016/j.jtemb.2022.126928.
(4) Herbst RA, Uter W, Pirker C, Geier J, Frosch PJ. Allergic and non-allergic periorbital dermatitis: patch test results of the Information Network of the Departments of Dermatology during a 5-year period. Contact Dermatitis. 2004 Jul;51(1):13-9. doi: 10.1111/j.0105-1873.2004.00334.x.
Abstract. Periorbital dermatitis is common and can be due to the external use of ophthalmic drugs. We evaluated patch test results of the Information Network of the Departments of Dermatology. During a 5-year period (1995-99), of a total 49,256 patch-tested patients, 1053 (2.1%) were eventually diagnosed as allergic periorbital contact dermatitis (APD) and 588 (1.2%) as non-allergic periorbital dermatitis (NAPD). Patient characteristics between APD, NAPD and other cases (OCs) differed with respect to sex (19.7% male in both periorbital groups versus 36.3% in OCs), atopic dermatitis (10.4% in APD versus 60.2% in NAPD versus 16.9% in OCs) and age, APD being substantially more often (68.2%) aged 40 and above than NAPD (52.6%). Several of the top allergens in OCs [such as fragrance mix, Myroxylon pereirae resin (balsam of Peru), lanolin alcohol and potassium dichromate] caused significantly fewer positive test reactions in both periorbital groups. In contrast, thimerosal, phenylmercuric acetate, sodium disulfite, gentamicin sulfate, phenylephrine hydrochloride and benzalkonium chloride tested positively significantly more often in APD but not in NAPD, verifying them as true ophthalmic allergens. Finally, in 42 cases (4%) of APD patients, additional allergens were identified by testing of the patients' own substances (mostly beta-blockers, oxybuprocaine and dexpanthenol), supporting the necessity of testing with ophthalmic drugs as is where individual substances are not readily available.
| Evaluate |

