| "Descrizione" by Nat45 (5786 pt) | 2025-Aug-05 21:02 |
Review Consensus: 10 Rating: 10 Number of users: 1
| Evaluation | N. Experts | Evaluation | N. Experts |
|---|---|---|---|
| 1 | 6 | ||
| 2 | 7 | ||
| 3 | 8 | ||
| 4 | 9 | ||
| 5 | 10 |
E553b or Talc is a chemical compound that is produced directly from talc, steatite, sepiolite, a pressed hydrated magnesium silicate, treated with hydrochloric acid, washed and dried. In its composition are available asbestos, amphiboles, chrysotile, aluminum.
The name defines the structure of the molecule:
- Talc. The name is derived from the Arabic word 'talq', meaning 'pure'. It is a reference to its white colour and the softness of the mineral.
The synthesis process takes place in different stages:
- Mother rock formation. Talc is formed from parent rocks rich in magnesium and silica. The mother rock can be dolomite or serpentine.
- Metamorphism. Over millions of years, these rocks undergo metamorphism, a process in which the mineral composition and structure of the rock are changed by heat and pressure. This process can lead to the formation of talc.
- Extraction and processing Once formed, talc is extracted from open-pit mines and then ground into a fine powder for use.
It comes in the form of a fine, odorless, tasteless, white non-sandy powder with a greasy feel to the touch.

What is it for?
Used in a variety of industries: paints, cosmetics, linoleum, textiles, rubber, paper, plastics, talc is a good modifier for elastomers, improves stiffness and barrier properties. Because of its high specific surface area characteristics, talc is used to dust sticky products, prevents agglomeration and improves handling. Talc has acid and fire resistance, insulation, high melting point, strong absorption strength and is chemically inert because of its crystallization due to its layered structure.
Pharmaceutical
Filling agent, coating of tablets Talc is a gliding excipient, in practice it increases the smoothness of the material in the tablet by reducing the friction between the particles. Typically the concentration is: 0.3-10 %w/w.
Cosmetics
Talc is a restricted ingredient as III/59 a Relevant Item in the Annexes of the European Cosmetics Regulation 1223/2009. Ingredient at risk: Talc: Hydrated magnesium silicate. (a) Powdery products intended to be used for children under 3 years of age (b) Other products. (a) Keep powder away from children's nose and mouth
- Abrasive agent. It contains abrasive particles to remove stains or biofilm that accumulate on the stratum corneum or teeth. Baking soda, kieselguhr, silica and many others have abrasive properties. Peeling or exfoliating products used in dermatology or cosmetic applications contain abrasive agents in the form of synthetic microspheres, however these microspheres or abrasive particles are not biodegradable and create pollution in aquatic ecosystems.
- Absorbent. Absorbs substances dispersed or dissolved in aqueous solutions, water/oil, oil/water.
- Anticaking agent. This compound facilitates free flow and prevents aggregation or clumping of substances in a formulation by reducing the tendency of certain particles to stick together.
- Bulking agent. It regulates the water content, dilutes other solids, can increase the volume of a product for better flow, acts as a buffer against organic acids, helps to keep the pH of the mixture within a certain level.
- Opacifying agent. It is useful into formulations that may be translucent or transparent to make them opaque and less permeable to light.
- Skin protectant. It creates a protective barrier on the skin to defend it from harmful substances, irritants, allergens, pathogens that can cause various inflammatory conditions. These products can also improve the natural skin barrier and in most cases more than one is needed to achieve an effective result.
- Slip modifier. It increases the spreadability of a product by helping other substances flow more smoothly and easily, without chemical reaction..
Food
Labeled with the number E553b, anti-caking agent, in the European food additives list.
Other uses
Rubber: filling agent (the dosage is 5% of the amount of poly (vinyl acetate)
And also used in printing inks, ceramics, cables, waterproof materials and others
Safety
In recent years, many manufacturers have removed asbestos (a known carcinogenic mineral) from the production of talcum powder because of the dangers that asbestos can create to human health, but some precautions still need to be observed: talcum powder must not come into contact with the horny layer when the latter is damaged.
Talc must comply with cosmetic regulations (Reg. EU 1223/2009) and therefore does not record the presence of asbestos.
Among the impurities present in talcum powder processing, aluminium is of some concern. Aluminium can interfere with different biological processes (cellular oxidative stress, calcium metabolism, etc.), so it can induce toxic effects in different organs and systems, and the nervous system is the main target of its toxicity.
Talc: What It Is and What It Contains
Talc is a natural mineral, chemically defined as hydrated magnesium silicate (Mg₃Si₄O₁₀(OH)₂). It does not contain aluminum in its basic formula.
Aluminum Risk in Talc
Pure talc: By itself, talc does NOT contain aluminum in its standard mineral structure.
Contaminations: However, as with all natural minerals, talc may contain impurities due to contamination from other minerals present in the deposits. In rare cases, there may be traces of aluminosilicates (that is, silicates containing aluminum) if the deposit is mixed or contaminated with minerals like kaolinite or mica.
Industrial or low-quality talc: This could have a higher chance of containing impurities, but cosmetic and pharmaceutical products are generally subject to strict controls that limit the presence of such substances.
Additives: In some cases, certain cosmetic powders may contain added aluminum compounds (e.g., aluminum silicate, aluminum oxide), but these must be clearly listed on the label as additional ingredients and are not present in pure talc.
Regulations and Safety
In Europe and many other countries, talc used in cosmetics is controlled and must be free from asbestos and significant contaminants, including aluminum-based ones.
The presence of aluminum in pure cosmetic talc is therefore negligible or absent, and does not represent a known risk in regulated products.
Conclusion
Pure talc does NOT contain aluminum.
If you choose reliable and regulated cosmetic products, the “aluminum” risk is practically nonexistent.
If you are unsure about a powder that contains “talc” among its ingredients, always check the INCI list to see if aluminum-based compounds have been added (e.g., Aluminum Silicate, Aluminum Starch Octenylsuccinate, etc.).
Studies
This study finds that exposure to talc in female genitals produces an increase in inflammation resulting in an increased risk of ovarian cancer (1).
Because of the contained asbestos, talc inhalation can cause pulmonary fibrosis in the form of granulomatous nodules called talcosis. Talc exposure has also been suggested as a causative factor in the development of ovarian carcinomas, gynecological cancers and mesothelioma (2).
This study assessed the risk of contracting asbestos-related disease from powdered cosmetic talcum users. The hypothetical treatment of this fiber as if it were an asbestos involves a risk of 9.6 × 10-7 (less than one in a million) (3).
Some cases of talc poisoning (4).
The most relevant studies on this ingredient have been selected with a summary of their contents:
Typical commercial product characteristics
| Appearance | White fine powder |
| Silicon Dioxide, W / % | ≥58.0 |
| Density | 2.7-2.8 g/cm3 |
| pH | 8.0-9.5 |
| Melting Point | 800ºC |
| LOI (at 1050℃) | 13--17% |
| Magnesium Oxide, W / % | ≥30.0 |
| Whiteness | ≥85.0 |
| Acid soluble substance (S04) / % | ≤1.5 |
| Loss on ignition/ % | ≤6.0 |
| Loss on dying / % | ≤0.5 |
| Asbestos | Free |
| Arsenic mg/kg | ≤3 |
| Pb mg/kg | ≤5 |
| Water soluble salt/ % | ≤0.1 |
| Heavy metals(Pb) mg/kg | ≤10 |
| Fineness(45μm), w / % | ≥98.0 |
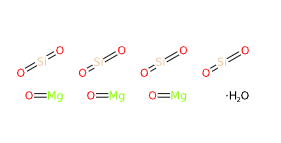 |  |
- Molecular Formula : Mg3(OH)2Si4O10 H2Mg3O12Si4 Mg3(Si4O10)(OH)2
- Linear Formula : 3MgO·4SiO2·H2O
- Molecular Weight : 379.259 g/mol
- CAS : 14807-96-6
- UNII 7SEV7J4R1U
- EC Number: 238-877-9
- DSSTox Substance ID: DTXSID5032109
- MDL number MFCD00792903
- PubChem Substance ID
- InChI=1S/3Mg.4O2Si.H2O.3O/c;;;4*1-3-2;;;;/h;;;;;;;1H2;;;
- InChl Key FPAFDBFIGPHWGO-UHFFFAOYSA-N
- SMILES O.O=[Mg].O=[Mg].O=[Mg].O=[Si]=O.O=[Si]=O.O=[Si]=O.O=[Si]=O
- IUPAC dioxosilane;oxomagnesium;hydrate
- ChEBI
Synonyms :
- Talc CP
- tris(@magnesium oxide) tetrakis(silica) hydrate
- tris(@magnesium oxide) tetrakis(silica) hydrate
- 3MgO.4O2Si.H2O
- E553b
References____________________________________________________________________
Abstract. Genital use of talcum powder and its associated risk of ovarian cancer is an important controversial topic. Epithelial ovarian cancer (EOC) cells are known to manifest a persistent prooxidant state. Here we demonstrated that talc induces significant changes in key redox enzymes and enhances the prooxidant state in normal and EOC cells. Using real-time reverse transcription polymerase chain reaction and enzyme-linked immunosorbent assay, levels of CA-125, caspase-3, nitrate/nitrite, and selected key redox enzymes, including myeloperoxidase (MPO), inducible nitric oxide synthase (iNOS), superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPX), and glutathione reductase (GSR), were determined. TaqMan genotype analysis utilizing the QuantStudio 12K Flex was used to assess single-nucleotide polymorphisms in genes corresponding to target enzymes. Cell proliferation was determined by MTT proliferation assay. In all talc-treated cells, there was a significant dose-dependent increase in prooxidant iNOS, nitrate/nitrite, and MPO with a concomitant decrease in antioxidants CAT, SOD, GSR, and GPX (P < .05). Remarkably, talc exposure induced specific point mutations that are known to alter the activity in some of these key enzymes. Talc exposure also resulted in a significant increase in inflammation as determined by increased tumor marker CA-125 (P < .05). More importantly, talc exposure significantly induced cell proliferation and decreased apoptosis in cancer cells and to a greater degree in normal cells (P < .05). These findings are the first to confirm the cellular effect of talc and provide a molecular mechanism to previous reports linking genital use to increased ovarian cancer risk.
Abstract. Background: Cosmetic talcum powder products have been used for decades. The inhalation of talc may cause lung fibrosis in the form of granulomatose nodules called talcosis. Exposure to talc has also been suggested as a causative factor in the development of ovarian carcinomas, gynecological tumors, and mesothelioma. Purpose: To investigate one historic brand of cosmetic talcum powder associated with mesothelioma in women. Methods: Transmission electron microscope (TEM) formvar-coated grids were prepared with concentrations of one brand of talcum powder directly, on filters, from air collections on filters in glovebox and simulated bathroom exposures and human fiber burden analyses. The grids were analyzed on an analytic TEM using energy-dispersive spectrometer (EDS) and selected-area electron diffraction (SAED) to determine asbestos fiber number and type. Results: This brand of talcum powder contained asbestos and the application of talcum powder released inhalable asbestos fibers. Lung and lymph node tissues removed at autopsy revealed pleural mesothelioma. Digestions of the tissues were found to contain anthophyllite and tremolite asbestos. Discussion: Through many applications of this particular brand of talcum powder, the deceased inhaled asbestos fibers, which then accumulated in her lungs and likely caused or contributed to her mesothelioma as well as other women with the same scenario.
(4) Pennycuff JF, Davenport A, Ellis J, Patberg E, Cwiak C. Talcum Powder Toxicosis in Pregnancy. AJP Rep. 2018 Oct;8(4):e384-e386. doi: 10.1055/s-0038-1676382.
Abstract. Background Pica is a relatively common phenomenon in pregnancy and typically includes consumption of nontoxic substances such as earth/clay, raw starches, and ice. Occasionally, substances may be toxic or have unintended consequences. Case A nulliparous woman presented to our facility complaining of numerous, vague symptoms that are common in pregnancy. She had multiple work-ups and an admission to our antepartum unit without clear etiology of her symptoms. Ultimately, she was diagnosed with talcum powder toxicosis secondary to talc ingestion as a coping mechanism for her anxiety, which was heightened in pregnancy. Conclusion This case highlights the importance of screening for mental health disorders, which may be exacerbated during the peripartum period. Patients' coping mechanisms for mental health disorders may have unintended consequences.
(5) Davies R, Skidmore JW, Griffiths DM, Moncrieff CB. Cytotoxicity of talc for macrophages in vitro. Food Chem Toxicol. 1983 Apr;21(2):201-7. doi: 10.1016/0278-6915(83)90237-5. PMID: 6682083.
Abstract. The cytotoxicity of seven specimens of respirable talc dust for mouse peritoneal macrophages in vitro was studied. All talcs showed modest but consistent macrophage cytotoxicity and would be expected to be fibrogenic in vivo. Available data suggest that under certain circumstances respirable talc can cause lung fibrosis in animal inhalation studies. As most of the talc specimens under investigation were of high purity it seemed unlikely that the cytotoxicity was due to the presence of small quantities of contaminating minerals.
| Evaluate |
