| "Descrizione" by A_Partyns (12948 pt) | 2023-Jul-18 19:12 |
Review Consensus: 10 Rating: 10 Number of users: 1
| Evaluation | N. Experts | Evaluation | N. Experts |
|---|---|---|---|
| 1 | 6 | ||
| 2 | 7 | ||
| 3 | 8 | ||
| 4 | 9 | ||
| 5 | 10 |
Pluronic™ F127 or Poloxamer 407 is a chemical compound, surfactant non ionic, copolymer consisting of three ethylene oxide blocks and one hydrophobic propylene oxide block, relatively non-toxic to cells at low concentrations.
"Pluronic 127" is a trade name for a specific type of polymer known as poloxamer 407. This name was given by the company that manufactures it, BASF. The term "Pluronic" is a registered trademark of BASF and is used for a range of polyethylene oxide-propylene oxide-polyethylene oxide (PEO-PPO-PEO) block polymers.
The numbering in the name "Pluronic 127" provides information about the structure of the polymer. The number "127" specifically indicates the length and proportion of the polyethylene oxide (PEO) and polypropylene oxide (PPO) blocks in the polymer. In general, the first number (or first two numbers) in a poloxamer's name indicates the length of the PPO block, while the last number multiplied by 10 indicates the weight percentage of PEO in the polymer.
In the case of Pluronic 127 (Poloxamer 407), the polymer has a central PPO block with a length of about 40 repeat units and PEO blocks on both sides with a length of about 70 repeat units each. The weight percentage of PEO in the polymer is 70%.
The name describes the structure of the molecule:
- Poloxamer is a type of non-ionic triblock copolymer consisting of a hydrophobic central polyoxypropylene (propylene oxide) chain flanked by two hydrophilic polyoxyethylene (ethylene oxide) chains.
- 407 refers to the specific type of poloxamer, which is defined by the length of the polyoxypropylene and polyoxyethylene chains. In the case of poloxamer 407, the central polyoxypropylene chain consists of approximately 56 propylene oxide units and each polyoxyethylene chain consists of approximately 101 ethylene oxide units.
The raw materials for the production of Poloxamer 407 are:
- Propylene oxide - This is a chemical compound used to form the central hydrophobic chain of polyoxypropylene. Propylene oxide is a colorless volatile liquid.
- Ethylene oxide - This is a chemical compound used to form the hydrophilic chains of polyoxyethylene.
It is obtained by polymerisation of ethylene oxide and propylene oxide in liquid form at a controlled temperature with a catalyst such as potassium hydroxide or sodium hydroxide. The synthesis process consists of the following steps:
It is obtained by polymerisation of ethylene oxide and propylene oxide in liquid form at a controlled temperature with a catalyst such as potassium hydroxide or sodium hydroxide. The synthesis process consists of the following steps:
- Reaction. Propylene oxide is reacted with a suitable initiator, such as propylene glycol, in the presence of a catalyst to form the polyoxypropylene core. The reaction takes place at high temperature and under pressure.
- Polymerisation. Ethylene oxide is added to the reaction and attaches to the ends of the polyoxypropylene core, forming polyoxyethylene building blocks. The reaction is catalysed and takes place under pressure and at high temperature. Polymerisation is stopped once the desired molecular weights for the polyoxypropylene and polyoxyethylene sections are reached.
- Purification. The resulting poloxamer is purified by a precipitation and filtration process. Ethylene oxide impurities should have been removed.
It comes in the form of fine white powder.

What it is used for and where
Cosmetics
Surfactant - Cleansing agent. Cosmetic products used to cleanse the skin utilise the surface-active action that produces a lowering of the surface tension of the stratum corneum, facilitating the removal of dirt and impurities.
Surfactant - Emulsifying agent. Emulsions are thermodynamically unstable and are used to soothe or soften the skin and emulsify, so they need a specific, stabilising ingredient. This ingredient forms a film, lowers the surface tension and makes two immiscible liquids miscible. A very important factor affecting the stability of the emulsion is the amount of the emulsifying agent. Emulsifiers have the property of reducing the oil/water or water/oil interfacial tension, improving the stability of the emulsion and also directly influencing the stability, sensory properties and surface tension of sunscreens by modulating the filmometric performance.
Cosmetic safety. In cosmetics, Poloxamer 407 is considered safe, however, as with any cosmetic ingredient, some people may be sensitive or have individual allergic reactions, so it is always advisable to test products on the skin before particularly intensive use.
Food
Among a number of natural and synthetic polymers used to form polymeric micelles, Poloxamer 407, a US Food and Drug Administration-approved polymer, is the most attractive due to its biocompatibility, biodegradability, and low toxicity (1).
Poloxamer 407 is used as an emulsifier and stabiliser in creams, sauces, ice cream, fruit drinks where it improves appearance, stability and consistency, promotes solubilisation of lipophilic compounds, improves distribution and bioavailability.
Medical
On polyoxamers, scientific research has identified a potential use, thanks to their low toxicity, solubilising capacity, compatibility with various excipients and biomolecules, as a biomaterial to obtain hydrogels for drug release and in particular Poloxamer 407 is used as a carrier, thanks to its amphiphilic nature, to improve the solubility of poorly water-soluble drugs with particular warning on the solubilisation of active ingredients.
It is used, due to its viscosity and ability to form a protective film on the ocular surface as an ocular lubricant in eye drops that help alleviate dry eye symptoms, improve comfort and as an artificial tear.
Poloxamer 407 is a cleaning agent that can remove plaque and debris from ocular surfaces, contact lenses or medical devices.
It helps protect cells during the freezing and thawing process by acting as a cellular cryopreservative, reducing cell damage and preserving cell viability.
It also has a function as a stabiliser for emulsions, suspensions and colloidal systems improving the stability of pharmaceutical formulations and preventing phase separation and a gelling function with the ability to form reversible gels at room temperature useful in ophthalmology.
It is a non-ionic surfactant that facilitates the emulsification and homogeneous distribution of ingredients in pharmaceutical formulations.
The safety and efficacy of this polymer as a temporary embolic agent were analysed and evaluated positively in this study on temporary vascular occlusion (1).
Poloxamer 407 shows some thermoreversible properties of extreme interest in optimising drug formulation (3).
Poloxamer 407 and vitamin E TPG (D-α-tocopheryl polyethylene glycol succinate) are polymers widely used as drug carriers and excipients to improve drug retention and stability times (4) and oral delivery of antibiofilm peptides (5).
An interesting potential application of Poloxamer 407 concerns 'phacoemulsification' (ultrasound) to protect the corneal endothelium (6).
Safety
Available data show that Poloxamers introduced into the body through different routes than dermal exposure have a rapid clearance from the body, suggesting that they are safe as used (7).

The most relevant studies on this chemical compound have been selected with a summary of their contents:
Optimal typical characteristics of Poloxamer 407 commercial product
| Clarity and color of solution | Clear and colorless |
| Acid | Red solution |
| Average molecular weight | 9480 g/mol-14500 g/mol |
| Ph value (25g/l in water) | 5.0-7.0 |
| Ph value (100g/l in water) | 5.0-7.0 |
| Oxyethylene | 71.5%-74.9% |
| Total ash | <0.4% |
| Residue on ignition | <0.30 |
| Insaturation | 0.031mEq/G-0.65mEq/ |
| Water | <0.75% |
| Heavy metals | <0.002% |
| BHT stabilizer | 50ppm-25ppm |
| Congealing point | 50°C-62°C |
| 1.4 dioxane | <5.0ppm |
| Ethylene oxide | <1.0ppm |
| Propylene oxide | <5.0ppm |
| Arsenic | <2ppm |
Synonyms:
Adeka Pluronic F 108 Antarox Cirrasol ALN-WS Detalan Epan 485 Epan 710 Epan 785 Ethylene oxide-propylene oxide block copolymer dipropylene glycol ether Ethylene oxide-propylene oxide block copolymer ether with ethylene glycol F-108 Lutrol F Pluracare L 61 Poloxalene Poloxalkol Poloxamene Poloxamer 188 Poly(ethylene oxide-co-propylene oxide) Polyoxamer Polyoxyethylene - polyoxypropylene block copolymer Polyoxyethylene - polyoxypropylene copolymer Tergitol XH Therabloat
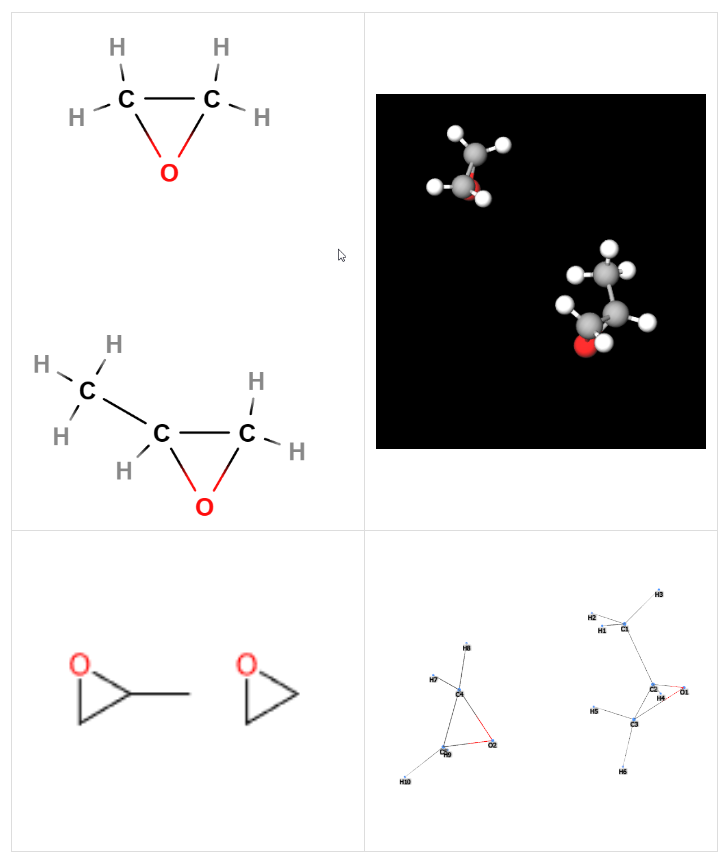
- Molecular Formula: C5H10O2
- Molecular Weight: 102,133
- CAS: 9003-11-6
- UNII
- EC Number: 923-642-1
- DSSTox Substance ID: DTXSID70872897 DTXSID4047597
- MDL number MFCD00082049
- PubChem Substance ID
- InChI=1S/C3H6O.C2H4O/c1-3-2-4-3;1-2-3-1/h3H,2H2,1H3;1-2H2
- InChl Key InChI=1S/C3H6O.C2H4O/c1-3-2-4-3;1-2-3-1/h3H,2H2,1H3;1-2H2
- SMILES CC1CO1.C1CO1
- IUPAC 2-methyloxirane;oxirane
- ChEBI 32026
(1) Saxena V, Hussain MD. Poloxamer 407/TPGS mixed micelles for delivery of gambogic acid to breast and multidrug-resistant cancer. Int J Nanomedicine. 2012;7:713-21. doi: 10.2147/IJN.S28745.
Abstract. Background: Delivery of a high concentration of anticancer drugs specifically to cancer cells remains the biggest challenge for the treatment of multidrug-resistant cancer. Poloxamers and D-α-Tocopheryl polyethylene glycol 1000 succinate (TPGS) are known inhibitors of P-glycoprotein (P-gp). Mixed micelles prepared from Poloxamer 407 and TPGS may increase the therapeutic efficacy of the drug by delivering high concentrations inside the cells and inhibiting P-gp. Gambogic acid (GA) is a naturally derived novel anticancer agent, but poor solubility and toxic side effects limit its use. In this study, we have developed Poloxamer 407 and TPGS mixed micelle-encapsulating GA for the treatment of breast and multidrug-resistant cancer.... Conclusion: This study suggests that Poloxamer 407/TPGS mixed micelles can be used as a delivery system for GA to treat breast and multidrug-resistant cancer.
(2) Raymond J, Metcalfe A, Salazkin I, Schwarz A. Temporary vascular occlusion with poloxamer 407. Biomaterials. 2004 Aug;25(18):3983-9. doi: 10.1016/j.biomaterials.2003.10.085.
Abstract. There is a need for safe and reversible occlusions during percutaneous endovascular procedures. Poloxamer 407 is a non-ionic surfactant with rapid reversible sol-gel transition behaviour. The safety and efficacy of this polymer as a temporary embolic agent was investigated. First, dissolution time after gelation of poloxamer was determined in an in vitro model. Then, transient poloxamer occlusion of renal and pulmonary arteries of seven dogs was followed by serial angiograms. Macroscopic and pathological changes were studied 1 week later. This experiment was repeated in similar arteries in one pig, and in auricular arteries of two rabbits. Poloxamer dissolution after in vitro polymerization was completed within 1-20 h, depending on concentrations. In vivo poloxamer 22% injections led to complete occlusion, followed by full recanalization within 10-90 min without complication. The only biochemical effect of poloxamer occlusions was transient elevation of triglyceride levels. There were no pathological abnormalities at 1 week. Poloxamer 407 could be used as an embolic material for temporary occlusions.
(3) Dumortier G, Grossiord JL, Agnely F, Chaumeil JC. A review of poloxamer 407 pharmaceutical and pharmacological characteristics. Pharm Res. 2006 Dec;23(12):2709-28. doi: 10.1007/s11095-006-9104-4.
Abstract. Poloxamer 407 copolymer (ethylene oxide and propylene oxide blocks) shows thermoreversible properties, which is of the utmost interest in optimising drug formulation (fluid state at room temperature facilitating administration and gel state above sol-gel transition temperature at body temperature promoting prolonged release of pharmacological agents). Pharmaceutical evaluation consists in determining the rheological behaviour (flow curve or oscillatory studies), sol-gel transition temperature, in vitro drug release using either synthetic or physiological membrane and (bio)adhesion characteristics. Poloxamer 407 formulations led to enhanced solubilisation of poorly water-soluble drugs and prolonged release profile for many galenic applications (e.g., oral, rectal, topical, ophthalmic, nasal and injectable preparations) but did not clearly show any relevant advantages when used alone. Combination with other excipients like Poloxamer 188 or mucoadhesive polymers promotes Poloxamer 407 action by optimising sol-gel transition temperature or increasing bioadhesive properties. Inclusion of liposomes or micro(nano)particles in Poloxamer 407 formulations offers interesting prospects, as well. Besides these promising data, Poloxamer 407 has been held responsible for lipidic profile alteration and possible renal toxicity, which compromises its development for parenteral applications. In addition, new findings have demonstrated immuno-modulation and cytotoxicity-promoting properties of Poloxamer 407 revealing significant pharmacological interest and, hence, human trials are in progress to specify these potential applications.
(4) Butt AM, Mohd Amin MC, Katas H. Synergistic effect of pH-responsive folate-functionalized poloxamer 407-TPGS-mixed micelles on targeted delivery of anticancer drugs. Int J Nanomedicine. 2015 Feb 13;10:1321-34. doi: 10.2147/IJN.S78438.
Abstract. Background: Doxorubicin (DOX), an anthracycline anticancer antibiotic, is used for treating various types of cancers. However, its use is associated with toxicity to normal cells and development of resistance due to overexpression of drug efflux pumps. Poloxamer 407 (P407) and vitamin E TPGS (D-α-tocopheryl polyethylene glycol succinate, TPGS) are widely used polymers as drug delivery carriers and excipients for enhancing the drug retention times and stability. TPGS reduces multidrug resistance, induces apoptosis, and shows selective anticancer activity against tumor cells. Keeping in view the problems, we designed a mixed micelle system encapsulating DOX comprising TPGS for its selective anticancer activity and P407 conjugated with folic acid (FA) for folate-mediated receptor targeting to cancer cells.....Conclusion: FA-P407-TPGS-DOX micelles show potential as a targeted nano-drug delivery system for DOX due to their multiple synergistic factors of selective anticancer activity, inhibition of multidrug resistance, and folate-mediated selective uptake.
(5) Bernegossi J, Calixto GM, Sanches PR, Fontana CR, Cilli EM, Garrido SS, Chorilli M. Peptide KSL-W-Loaded Mucoadhesive Liquid Crystalline Vehicle as an Alternative Treatment for Multispecies Oral Biofilm. Molecules. 2015 Dec 25;21(1):E37. doi: 10.3390/molecules21010037.
Abstract. Decapeptide KSL-W shows antibacterial activities and can be used in the oral cavity, however, it is easily degraded in aqueous solution and eliminated. Therefore, we aimed to develop liquid crystalline systems (F1 and F2) for KSL-W buccal administration to treat multispecies oral biofilms. The systems were prepared with oleic acid, polyoxypropylene (5) polyoxyethylene (20) cetyl alcohol (PPG-5-CETETH-20), and a 1% poloxamer 407 dispersion as the oil phase (OP), surfactant (S), and aqueous phase (AP), respectively. We characterized them using polarized light microscopy (PLM), small-angle X-ray scattering (SAXS), rheology, and in vitro bioadhesion, and performed in vitro biological analysis. PLM showed isotropy (F1) or anisotropy with lamellar mesophases (F2), confirmed by peak ratio quantification using SAXS. Rheological tests demonstrated that F1 exhibited Newtonian behavior but not F2, which showed a structured AP concentration-dependent system. Bioadhesion studies revealed an AP concentration-dependent increase in the system's bioadhesiveness (F2 = 15.50 ± 1.00 mN·s) to bovine teeth blocks. Antimicrobial testing revealed 100% inhibition of multispecies oral biofilm growth after KSL-W administration, which was incorporated in the F2 aqueous phase at a concentration of 1 mg/mL. Our results suggest that this system could serve as a potential vehicle for buccal administration of antibiofilm peptides.
(6) Galvis V, Tello A, Carreño NI, Berrospi RD, Niño CA. Potential use of thermoreversible hydrogel (poloxamer 407) to protect the corneal endothelium and the posterior capsule during phacoemulsification. J Cataract Refract Surg. 2019 Mar;45(3):389. doi: 10.1016/j.jcrs.2018.10.051.
(7) Singh-Joy SD, McLain VC. Safety assessment of poloxamers 101, 105, 108, 122, 123, 124, 181, 182, 183, 184, 185, 188, 212, 215, 217, 231, 234, 235, 237, 238, 282, 284, 288, 331, 333, 334, 335, 338, 401, 402, 403, and 407, poloxamer 105 benzoate, and poloxamer 182 dibenzoate as used in cosmetics. Int J Toxicol. 2008;27 Suppl 2:93-128. doi: 10.1080/10915810802244595.
| Evaluate |

