Butylene Glycol
Review Consensus: 10 Rating: 10 Number of users: 1
| "Descrizione" by A_Partyns (12948 pt) | 2023-Jul-28 15:25 |
Review Consensus: 10 Rating: 10 Number of users: 1
| Evaluation | N. Experts | Evaluation | N. Experts |
|---|---|---|---|
| 1 | 6 | ||
| 2 | 7 | ||
| 3 | 8 | ||
| 4 | 9 | ||
| 5 | 10 |
Butylene Glycol (1,3-butanediol o 1,3-Butylene Glycol) is an organic alcohol, an organic chemical of the C4 platform, butanediol glycol. It is a compound of butanediol with two hydroxy groups in positions 1 and 3 derived from a butane hydride.
The name describes the structure of the molecule
- Butylene - Indicates a carbon chain with four atoms.
- Glycol - Denotes the presence of two alcohol (–OH) groups in the molecule.
Description of raw materials used in production
- Crude oil - Butylene glycol is often derived from crude oil through cracking processes and subsequent chemical reactions.
Synthesis process
- Petroleum cracking - To obtain lighter fractions like propene.
- Hydration of propene - This process involves adding water to propene to form propanol.
- Conversion of propanol to butanediol - Through reaction and distillation processes, propanol can be converted into 1,3-butylene glycol.
It appears as a viscous, odourless liquid with a slightly bitter taste or as a fine white or yellowish powder. Highly soluble in water..

What it is used for and where
Cosmetics
- Humectant. Hygroscopic compound used to minimise water loss in the skin and to prevent it from drying out by facilitating faster and greater absorption of water into the stratum corneum of the epidermis. The epidermis is the most superficial of the three layers that make up human skin (epidermis, dermis and hypodermis) and is the layer that maintains hydration in all three layers. In turn, the epidermis is composed of five layers: horny, the most superficial, granular, spinous, shiny, and basal. Humectants have the ability to retain the water they attract from the air in the stratum corneum and have the function of moisturising the skin. They are best used before emollients, which are oil-based.
- Fragrance. It plays a decisive and important role in the formulation of cosmetic products as it provides the possibility of enhancing, masking or adding fragrance to the final product, increasing its marketability. It is able to create a perceptible pleasant odour, masking a bad smell. The consumer always expects to find a pleasant or distinctive scent in a cosmetic product.
- Skin conditioning agent. It is the mainstay of topical skin treatment as it has the function of restoring, increasing or improving skin tolerance to external factors, including melanocyte tolerance. The most important function of the conditioning agent is to prevent skin dehydration, but the subject is rather complex and involves emollients and humectants that can be added in the formulation.
- Solvent. It is the substance for dissolving or dispersing surfactants, oils, dyes, flavourings, bactericidal preservatives in solution.In fact, it dissolves other components present in a cosmetic formulation. Solvents are generally liquid (aqueous and non-aqueous).
- Viscosity control agent. It controls and adapts, Increasing or decreasing, viscosity to the required level for optimal chemical and physical stability of the product and dosage in gels, suspensions, emulsions, solutions.
Food
Flavouring agent. Antibacterial agent for cheese, meat, etc.
Medical
Intermediate chemical compound.
Other uses
- Used in polyester plasticisers, raw material and co-monomer of unsaturated polyester resin, in polyurethane resins. Industrial dehydrating agent. Moisturizer and softener for textiles, tobacco and paper. Chemical intermediate in dyes.
Typical optimal commercial product characteristics 1,3-Butylene Glycol
| Appearance | Viscous liquid or fine white powder |
| Boiling Point | 207.0±0.0 °C at 760 mmHg |
| Melting Point | -54ºC |
| Flash Point | 121.1±0.0 °C |
| Density | 1.0±0.1 g/cm3 |
| Relative density (20℃/ 20℃, g/cm3) | 1.004-1.007 |
| Purity (%) | ≥99.5 |
| Water content | ≤0.5% |
| PSA | 40.46000 |
| Color APHA | ≤10 |
| Vapour density | 3.1 (20 °C) |
| Vapour Pressure | 0.1±0.8 mmHg at 25°C |
| Index of Refraction | 1.4390-1.4410 |
| Refractive Rate | n20/D1.44 |
 |  |
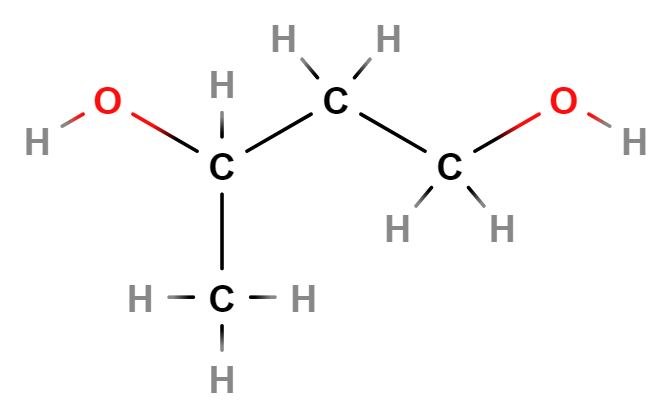 | 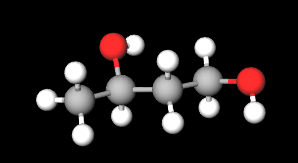 |
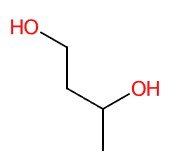
- Molecular Formula: C4H10O2
- Linear Formula CH3CH(OH)CH2CH2OH
- Molecular Weight: 90.122 g/mol
- Exact Mass 90.068077
- CAS: 107-88-0 18826-95-4 6290-03-5 817176-75-3
- UNII 3XUS85K0RA
- EC Number: 203-529-7 228-532-0
- PubChem Substance ID 57648493
- DSSTox Substance ID DTXSID8026773
- IUPAC butane-1,3-diol
- InChI=1S/C4H10O2/c1-4(6)2-3-5/h4-6H,2-3H2,1H3
- InChl Key PUPZLCDOIYMWBV-UHFFFAOYSA-N
- SMILES CC(CCO)O
- MDL number MFCD00004554
- Beilstein Registry Number 1731276
- ICSC 1182
- NSC 402145 6966
- RTECS EK0440000
Synonyms:
- (±)-1,3-Butanediol
- 1,3-Dihydroxybutane
- Methyltrimethylene glycol
- 1,3-Butandiol
- 1-Methyl-1,3-propanediol
- beta-Butylene glycol
- 1,3 Butylene glycol
- 1,3-BUTANEDIOL
- Butane-1,3-diol
- 1,3-Butylenglykol
- (RS)-1,3-Butandiol
- 1,3-Butanodiol
- (+/-)-1,3-Butanediol
- Caswell No. 128GG
- 1,3-Butylene glycol
- 1,3-Butanediol, (R)-
- 1,3-Butanediol, (S)-
- S-(+)-1,3-Butanediol
- (R)-(-)-1,3-Butylene Glycol
- Aliphatic diol
- b-Butylene glycol
| Evaluate |

