| "Descrizione" by Frank123 (12058 pt) | 2023-Sep-26 16:18 |
Review Consensus: 9 Rating: 9 Number of users: 1
| Evaluation | N. Experts | Evaluation | N. Experts |
|---|---|---|---|
| 1 | 6 | ||
| 2 | 7 | ||
| 3 | 8 | ||
| 4 | 9 | ||
| 5 | 10 |
Ferrous Sulfate is an inorganic compound made up of iron, sulfur, and oxygen, a salt derived from sulfuric acid. It is made up of bivalent iron (Fe^2+) and the sulfate anion (SO₄^2−).
Description of raw materials used in production.
- The primary raw materials for producing ferrous sulfate are iron and sulfuric acid.
Step-by-step summary of the industrial chemical synthesis process.
- Iron is exposed to diluted sulfuric acid.
- A reaction ensues where ferrous sulfate and hydrogen gas are produced.
- The resulting solution is evaporated to yield ferrous sulfate crystals.
- The crystals are subsequently dried.
Ferrous sulfate typically appears as green crystals or as a greenish-blue crystalline powder.

What it is for and where
Cosmetics
Cosmetic astringent. This ingredient exerts a direct effect on the skin by tightening dilated pores by contracting stratum corneum cells and removing superfluous oil.
Food
Ferrous sulfate is often added to foods as an iron fortificant. It's common in products like breakfast cereals, flour, and bread. Its addition helps prevent iron deficiency, one of the most common nutritional deficiencies globally.
Prevention of Anemia - Iron deficiency can lead to anemia, a condition where the body doesn't have enough healthy red blood cells. Ferrous sulfate helps prevent this condition.
Support Cognitive Function - Iron is essential for cognitive function and brain development.
Energy and Metabolism - Iron plays a key role in metabolism and energy production in the body.
Safety
While ferrous sulfate has many benefits, an excess can be toxic. It's essential to consume iron in appropriate amounts and under the supervision of a healthcare professional.
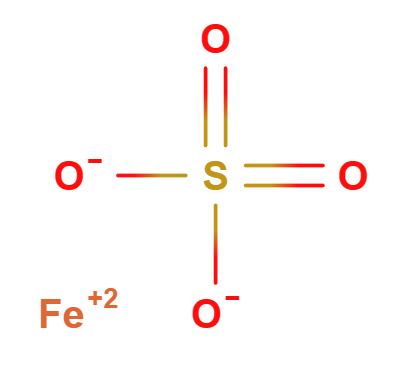 |  |
- Molecular Formula FeO4S
- Molecular Weight 151.91 g/mol
- CAS 7720-78-7
- UNII 2IDP3X9OUD
- EC Number 231-753-5
- DTXSID0029688
- Nikkaji J3.753B
Synonyms:
Iron sulfate
Bibliografia_____________________________________________________________________
(1) Zoe Tolkien Z, Stecher L, Mander AP, Pereira DI, Powell JJ. Ferrous sulfate supplementation causes significant gastrointestinal side-effects in adults: a systematic review and meta-analysis. PLoS One. 2015 Feb 20;10(2):e0117383. doi: 10.1371/journal.pone.0117383.
Abstract. Background: The tolerability of oral iron supplementation for the treatment of iron deficiency anemia is disputed. Objective: Our aim was to quantify the odds of GI side-effects in adults related to current gold standard oral iron therapy, namely ferrous sulfate. Methods: Systematic review and meta-analysis of randomized controlled trials (RCTs) evaluating GI side-effects that included ferrous sulfate and a comparator that was either placebo or intravenous (i.v.) iron. Random effects meta-analysis modelling was undertaken and study heterogeneity was summarised using I2 statistics....Conclusions: Our meta-analysis confirms that ferrous sulfate is associated with a significant increase in gastrointestinal-specific side-effects but does not find a relationship with dose.
| Evaluate |

