| "Descrizione" by FRanier (9971 pt) | 2024-Oct-05 12:23 |
Review Consensus: 10 Rating: 10 Number of users: 1
| Evaluation | N. Experts | Evaluation | N. Experts |
|---|---|---|---|
| 1 | 6 | ||
| 2 | 7 | ||
| 3 | 8 | ||
| 4 | 9 | ||
| 5 | 10 |
Propyl sodium parahydroxybenzoate, commonly known as sodium propylparaben, is a sodium salt of propylparaben, which belongs to the paraben family. It is used as a preservative in cosmetics, personal care products, and pharmaceuticals due to its antimicrobial properties, particularly its ability to prevent the growth of mold, yeast, and bacteria. This ensures that products maintain their quality and shelf life by preventing microbial contamination.
Chemical Composition and Structure
Propyl sodium parahydroxybenzoate is a paraben ester formed by the reaction of parahydroxybenzoic acid with propanol, then neutralized with sodium hydroxide. Its chemical structure consists of a benzoic acid core with a propyl group attached, which contributes to its antimicrobial efficacy. The sodium salt form improves its solubility in water, making it suitable for use in aqueous formulations.
Physical Properties
Propyl sodium parahydroxybenzoate typically appears as a white crystalline powder. It is water-soluble, which makes it ideal for use in water-based formulations. It is stable over a wide range of pH levels and temperatures, providing long-lasting preservative effects in a variety of products.
The name describes the structure of the molecule:
- Sodium indicates the presence of a sodium ion. Sodium is an alkali metal, and in this context, it's used to form a salt with parahydroxybenzoic acid, making the molecule water-soluble.
- Propyl indicates the propyl group, an alkyl chain with three carbon atoms. It's the lipophilic (or hydrophobic) component of the molecule, meaning it has a preference for oily or fatty substances.
- Parahydroxybenzoate is the main part of the molecule and indicates that it's a derivative of parahydroxybenzoic acid, a type of benzoic acid that has a hydroxy group in the para position on the benzene ring.
Raw Materials Used in Production.
Propyl parahydroxybenzoic acid is derived from benzoic acid, which can be sourced from benzoin or chemically synthesized.
Step-by-step Summary of Industrial Production Process.
- Synthesis of propyl parahydroxybenzoic acid. Benzoic acid is reacted with propyl alcohol to yield propyl parahydroxybenzoate ester.
- Neutralization. The ester is then neutralized with sodium hydroxide (NaOH) to form the salt, Sodium Propyl Parahydroxybenzoate.
- Purification. The product is purified through various methods such as filtration and crystallization to remove impurities.
Form and Color.
It appears as a white or almost white crystalline powder.
What it is for and where
Cosmetics
It is a restricted ingredient as V/12 a Relevant Item in the Annexes of the European Cosmetics Regulation 1223/2009. Substance or ingredient reported: Butyl 4-hydroxybenzoate and its salts Propyl 4-hydroxybenzoate and its salts
- Maximum concentration in ready for use preparation - 0,14% (as acid) for the sum of the individual concentrations - 0,8% (as acid) for mixtures of substances mentioned in entry 12 and 12a, here the sum of the individual concentrations of butyl and propylparaben and their salts does not exceed 0,14%
- Other Not to be used in leave-on products designed for application on the nappy area of children under three years of age.
- Wording of conditions of use and warnings For leave-on products designed for children under three years of age: "Do not use on the nappy area"
Preservative. Any product containing organic, inorganic compounds, water, needs to be preserved from microbial contamination. Preservatives act against the development of harmful microorganisms and against oxidation of the product.
Commercial Applications
Cosmetics Industry. Sodium Propylparaben is a preservative widely used in cosmetics and skincare products. It helps prevent the growth of microorganisms, thus extending product shelf life.
Food Industry. It's also utilized as a preservative in certain food products to prevent the growth of bacteria, molds, and yeasts.
Medicine. It may be used in some pharmaceutical formulations as a preservative.
Safety
11-6-2019 I had written to the European Commission's Directorate for Health and Food Safety (DG SANTE) reiterating doubts about the safety of parabens and E11 titanium dioxide. Finally, also from this body came the answer that clarifies all doubts:
"Regarding the use of methyl- and propylparaben as excipients in oral medicinal products for human use, I would advise you to look at the information provided by the European Medicines Agency at https://www.ema.europa.eu/en/use-methyl-propylparaben-excipients-human-medicinal-products-oral-use This discussion paper deals with methyl- and propylparaben, as these are the parabens predominantly used in oral pharmaceutical formulations. The focus of this paper is on possible endocrine disrupting effects in humans.
Parabens are components discussed and on whose safety many doubts have been raised, especially for the damage they would produce to the aquatic environment where they are discharged after use (1).
Parabens can contribute to obesity (2).
Parabens are preservative chemical compounds that have been the subject of attention in the scientific literature as possible endocrine disruptors (particularly propylparaben and butylparaben), i.e. with the possibility of damaging the hormone-producing glands in our bodies, particularly in the breasts. The 2004 study by Darbre et al. showed that parabens remain in our bodies as intact esters (2). Following this study, some of the scientific literature in 2005 and 2006 cast doubt on Darbre's conclusions and claimed they were limited. However, both the US FDA and the European SCCP authorised in 2006 the use of a single paraben in cosmetic products at a concentration of 0.4% and the use of total parabens at a concentration of 0.8%. However, there is no shortage of studies that consider the restrictions unnecessary: M. G. Kirchhof et al. in 2013 found that parabens are among the safest and most well-tolerated preservatives and that current data do not support drastic regulations or personal exposure restrictions. Darbre in 2014 published a further study in which he showed how parabens can cause DNA damage (3).
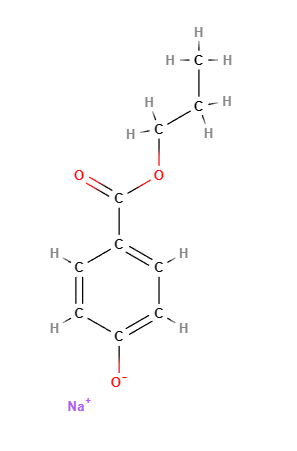 | 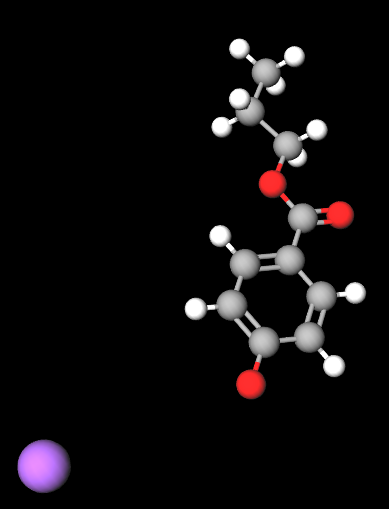 |
- Molecular Formula C10H11NaO3
- Molecular Weight 202.185 g/mol
- CAS 35285-69-9
- UNII 625NNB0G9N
- DTXSID3042348
- Nikkaji J42.046H
Synonyms :
sodium propyl p-hydroxybenzoate
Benzoic acid, 4-hydroxy-, propyl ester, sodium salt
Sodium 4-propoxycarbonylphenoxide
Sodium propylparaben
Propylparaben sodium
EINECS 252-488-1
sodium;4-propoxycarbonylphenolate
Sodium Propyl Parahydroxybenzoate
sodium;4-propoxycarbonylphenolate
Sodium propyl hydroxybenzoate
Sodium Propyl Parahydroxybenzoate
References___________________________________________________________________
(1) Terasaki M, Abe R, Makino M, Tatarazako N. Chronic toxicity of parabens and their chlorinated by-products in Ceriodaphnia dubia. Environ Toxicol. 2015 May-Jun;30(6):664-73. doi: 10.1002/tox.21944. Epub 2013 Dec 27. PMID: 24376163.
Popa DS, Bolfa P, Kiss B, Vlase L, Păltinean R, Pop A, Cătoi C, Crişan G, Loghin F. Influence of Genista tinctoria L. or methylparaben on subchronic toxicity of bisphenol A in rats. Biomed Environ Sci. 2014 Feb;27(2):85-96. doi: 10.3967/bes2014.021.
(2) Hu P, Chen X, Whitener RJ, Boder ET, Jones JO, Porollo A, Chen J, Zhao L. Effects of parabens on adipocyte differentiation. Toxicol Sci. 2013 Jan;131(1):56-70. doi: 10.1093/toxsci/kfs262.
Abstract. Parabens are a group of alkyl esters of p-hydroxybenzoic acid that include methylparaben, ethylparaben, propylparaben, butylparaben, and benzylparaben. Paraben esters and their salts are widely used as preservatives in cosmetics, toiletries, food, and pharmaceuticals. Humans are exposed to parabens through the use of such products from dermal contact, ingestion, and inhalation. However, research on the effects of parabens on health is limited, and the effects of parabens on adipogenesis have not been systematically studied. Here, we report that (1) parabens promote adipogenesis (or adipocyte differentiation) in murine 3T3-L1 cells, as revealed by adipocyte morphology, lipid accumulation, and mRNA expression of adipocyte-specific markers; (2) the adipogenic potency of parabens is increased with increasing length of the linear alkyl chain in the following potency ranking order: methyl- < ethyl- < propyl- < butylparaben. The extension of the linear alkyl chain with an aromatic ring in benzylparaben further augments the adipogenic ability, whereas 4-hydroxybenzoic acid, the common metabolite of all parabens, and the structurally related benzoic acid (without the OH group) are inactive in promoting 3T3-L1 adipocyte differentiation; (3) parabens activate glucocorticoid receptor and/or peroxisome proliferator-activated receptor γ in 3T3-L1 preadipocytes; however, no direct binding to, or modulation of, the ligand binding domain of the glucocorticoid receptor by parabens was detected by glucocorticoid receptor competitor assays; and lastly, (4) parabens, butyl- and benzylparaben in particular, also promote adipose conversion of human adipose-derived multipotent stromal cells. Our results suggest that parabens may contribute to obesity epidemic, and the role of parabens in adipogenesis in vivo needs to be examined further.
(3) Darbre PD, Harvey PW. Parabens can enable hallmarks and characteristics of cancer in human breast epithelial cells: a review of the literature with reference to new exposure data and regulatory status. J Appl Toxicol. 2014 Sep;34(9):925-38. doi: 10.1002/jat.3027.
| Evaluate |

