Check the ingredients!
... live healthy!


| "Descrizione" by Al222 (19776 pt) | 2024-Feb-01 18:58 |
Zinc gluconate is a chemical compound derived from zinc and gluconic acid.
The name describes the structure of the molecule:
Zinc. A chemical element with the symbol Zn and atomic number 30. Zinc is a metal, essential for numerous biological processes, including immune function, DNA synthesis, cell division, and wound healing. It acts as a cofactor for various enzymes, supporting metabolism and antioxidant activity.
Gluconate. A salt or ester of gluconic acid, which is derived from the fermentation of glucose. Gluconate acts as a chelating agent, binding metal ions such as zinc. This chelated form facilitates the absorption of zinc in the body, improving its bioavailability compared to other forms of zinc.
Zinc gluconate is synthesized industrially through a simple chemical reaction where gluconic acid reacts with zinc oxide. The process typically involves the following steps.
Form and Color
Commonly appears as a white powder or granules

What it is for and where
Dietary Supplements. Zinc gluconate is often used in dietary supplements as a source of zinc, an essential mineral for the immune system, cell repair, and metabolism
Oral Health. Found in some mouthwashes and toothpastes for its ability to prevent plaque formation and bad breath
Safety
Generally considered safe when taken in recommended doses, but excess zinc can cause side effects such as nausea and gastrointestinal upset
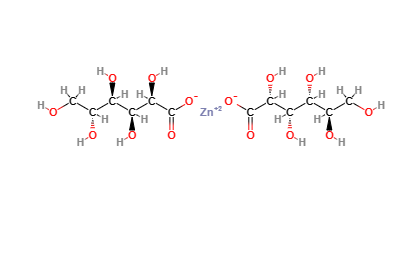 |  |
Molecular Formula C12H22O14Zn
Molecular Weight 455.7 g/mol
CAS 4468-02-4
UNII U6WSN5SQ1Z
EC Number 224-736-9
Synonyms
boron gluconate
Bibliografia_____________________________________________________________________
Siepmann M, Spank S, Kluge A, Schappach A, Kirch W. The pharmacokinetics of zinc from zinc gluconate: a comparison with zinc oxide in healthy men. Int J Clin Pharmacol Ther. 2005 Dec;43(12):562-5. doi: 10.5414/cpp43562. PMID: 16372518.
Abstract. Objective: Zinc supplementation is beneficial in some clinical conditions such as age-related macula degeneration (AMD). It has been suggested that zinc absorption is influenced by the form in which zinc is ingested. Therefore, the pharmacokinetics of zinc gluconate (organic) were compared with those of zinc oxide (inorganic)....Results: C(max) was found 18.3% (10.3 - 26.3%) higher following multiple-dose administration of zinc gluconate as compared to zinc oxide (mean; 0.95% confidence interval of the relative differences between both treatment conditions; p < 0.05). AUC(0-24h) was noted 8.1% (1.9 - 14.3%) higher after zinc was given as zinc gluconate when compared to zinc oxide (p < 0.05) whereas t(max) did not differ between both treatment conditions.
Marshall S. Zinc gluconate and the common cold. Review of randomized controlled trials. Can Fam Physician. 1998 May;44:1037-42.
Abstract. Objective: To examine the evidence of seven randomized controlled trials (RCT) on the therapeutic effectiveness of zinc gluconate lozenges for treating the common cold. Conclusion: Evidence supports use of zinc gluconate lozenges for reducing the symptoms and duration of the common cold, but the side effects, bad taste, and therapeutic protocol might limit patient compliance.
| Evaluate |