| "Descrizione" by Al222 (20718 pt) | 2024-May-29 09:16 |
Natural Yellow 3 is a chemical compound, a synthetic dye with curcumin (an orange-yellow polyphenolic flavonoid and the main constituent of the rhizome of the spice turmeric (Curcuma longa L. family Zingiberaceae) and is known as CI 75300.
What it is used for and where
Cosmetics
Restricted cosmetic ingredient as IV/114 a Relevant Item in the Annexes of the European Cosmetics Regulation 1223/2009. Substance or ingredient reported:
Curcumins. Wording of conditions of use and warnings: purity criteria as set out in Commission Directive 95/45/EC (E 100)
Cosmetics - INCI Functions
Colorant. This ingredient has the function of colouring the solution in which it is inserted in a temporary, semi-permanent or permanent manner, either alone or in the presence of the complementary components added for colouring.
___
Curcumin is a polyphenol with potent anti-inflammatory and antioxidant properties, which have been extensively studied in pharmacology and cosmetic science. The molecular structure of curcumin allows it to scavenge free radicals, thereby reducing oxidative stress, a contributing factor to cellular aging and pathology. Additionally, curcumin inhibits several molecules that play a significant role in the inflammation pathway, such as NF-kB, a protein complex that controls DNA transcription and cell survival.
Industrial Production Process
- Preparation of reagents. The main raw materials include the root of Curcuma longa (turmeric) and organic solvents such as ethanol or acetone.
- Harvesting and cleaning. The turmeric roots are harvested and cleaned to remove impurities and debris.
- Drying. The cleaned roots are dried at controlled temperatures to reduce moisture content and facilitate curcuminoid extraction.
- Grinding. The dried roots are ground into a fine powder to increase the contact surface area for extraction.
- Extraction. The turmeric powder is treated with a suitable solvent (ethanol, acetone, or a solvent mixture) to extract the curcuminoid compounds, primarily curcumin. Extraction can be done through maceration, infusion, or solvent extraction.
- Filtration. The extracted mixture is filtered to remove solid residues and obtain a clear solution containing curcumin.
- Concentration. The coloring solution is concentrated through vacuum evaporation to reduce the volume and increase the concentration of curcumin.
- Purification. The concentrated solution may be further purified using techniques such as chromatography to remove unwanted impurities and improve color quality.
- Crystallization. The purified curcumin is crystallized to obtain a high-purity product.
- Stabilization. The crystallized curcumin is stabilized with antioxidants like BHT (butylated hydroxytoluene) to prevent oxidation and maintain color stability during transportation and storage.
- Drying. The purified product is dried at controlled temperatures to obtain a fine curcumin powder.
- Quality control. The curcumin undergoes rigorous quality testing to ensure it meets standards for purity, color intensity, and safety. These tests include chemical analysis, spectroscopy, and microbiological testing.
Cosmetics
Restricted cosmetic ingredient as IV/114 a Relevant Item in the Annexes of the European Cosmetics Regulation 1223/2009. Substance or ingredient reported:
Curcumins. Wording of conditions of use and warnings: purity criteria as set out in Commission Directive 95/45/EC (E 100)
Cosmetics - INCI Functions
Antioxidant agent. Ingredient that counteracts oxidative stress and prevents cell damage. Free radicals, pathological inflammatory processes, reactive nitrogen species and reactive oxygen species are responsible for the ageing process and many diseases caused by oxidation.
Colorant. This ingredient has the function of colouring the solution in which it is inserted in a temporary, semi-permanent or permanent manner, either alone or in the presence of the complementary components added for colouring.
Medicine
In dermatological and cosmetic formulations, curcumin is valued not only for its pigment but also for its therapeutic effects on the skin. It has been shown to enhance wound healing by modulating collagen and decreasing reactive oxygen species. In anti-aging products, curcumin's ability to mitigate oxidative damage positions it as a beneficial ingredient in creams and serums designed to combat signs of aging. Furthermore, its anti-inflammatory properties make it a suitable component in treatments for skin conditions such as psoriasis and acne.
From a biochemical perspective, the therapeutic potentials of curcumin arise from its interaction with a variety of molecular targets involved in inflammation and oxidation. For instance, the modulation of cytokine production through the inhibition of the transcription factor NF-kB underlines the compound’s mechanism in treating inflammatory skin disorders.
Food
Labelled as E100 in the list of food additives dyes.
It is used as a spice in the kitchen especially in India.
Studies
There is a lot of positive scientific literature on Curcumin. It has anti-inflammatory effects that retard liver fibrosis and cirrhosis at the indicated doses (experiments on laboratory animals) (1) and in vivo (2).
Curcumin has known and explicit biological antitumor properties. It exerts an anti-proliferative and pro-apoptotic effect in tumor cells (3).
In healthy middle-aged and elderly adults, 12 weeks of curcumin supplementation (2000 mg / day Longvida®; n = 20) improved the endothelial function of the artery by increasing the vascular bioavailability of nitric oxide and reduced oxidative stress (4 ).
Curcumin exercises neuroprotective actions, in a model of cerebral ischemia, which are probably related to its anti-inflammatory activity (5).
Safety
Be careful not to exceed the doses recommended by your doctor.
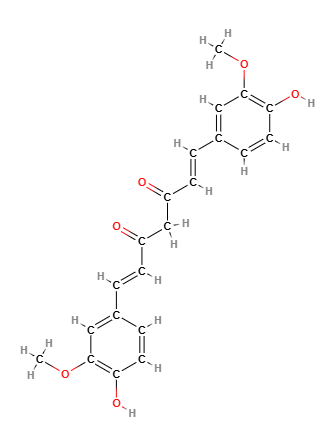 | 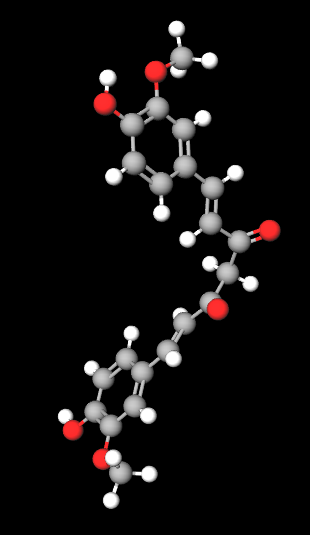 |
Molecular formula C21H20O6
Molecular weight 368.38
CAS 458-37-7
UNII IT942ZTH98
DTXSID8031077
References_____________________________________________________________________
(1) Fu Y, Zheng S, Lin J, Ryerse J, Chen A. Curcumin protects the rat liver from CCl4-caused injury and fibrogenesis by attenuating oxidative stress and suppressing inflammation. Mol Pharmacol. 2008 Feb;73(2):399-409. doi: 10.1124/mol.107.039818. E
Abstract. We previously demonstrated that curcumin, a polyphenolic antioxidant purified from turmeric, up-regulated peroxisome proliferator-activated receptor (PPAR)-gamma gene expression and stimulated its signaling, leading to the inhibition of activation of hepatic stellate cells (HSC) in vitro. The current study evaluates the in vivo role of curcumin in protecting the liver against injury and fibrogenesis caused by carbon tetrachloride (CCl(4)) in rats and further explores the underlying mechanisms. We hypothesize that curcumin might protect the liver from CCl(4)-caused injury and fibrogenesis by attenuating oxidative stress, suppressing inflammation, and inhibiting activation of HSC. This report demonstrates that curcumin significantly protects the liver from injury by reducing the activities of serum aspartate aminotransferase, alanine aminotransferase, and alkaline phosphatase, and by improving the histological architecture of the liver. In addition, curcumin attenuates oxidative stress by increasing the content of hepatic glutathione, leading to the reduction in the level of lipid hydroperoxide. Curcumin dramatically suppresses inflammation by reducing levels of inflammatory cytokines, including interferon-gamma, tumor necrosis factor-alpha, and interleukin-6. Furthermore, curcumin inhibits HSC activation by elevating the level of PPARgamma and reducing the abundance of platelet-derived growth factor, transforming growth factor-beta, their receptors, and type I collagen. This study demonstrates that curcumin protects the rat liver from CCl(4)-caused injury and fibrogenesis by suppressing hepatic inflammation, attenuating hepatic oxidative stress and inhibiting HSC activation. These results confirm and extend our prior in vitro observations and provide novel insights into the mechanisms of curcumin in the protection of the liver. Our results suggest that curcumin might be a therapeutic antifibrotic agent for the treatment of hepatic fibrosis.
(2) Chen YN, Hsu SL, Liao MY, Liu YT, Lai CH, Chen JF, Nguyen MT, Su YH, Chen ST, Wu LC. Ameliorative Effect of Curcumin-Encapsulated Hyaluronic Acid-PLA Nanoparticles on Thioacetamide-Induced Murine Hepatic Fibrosis. Int J Environ Res Public Health. 2016 Dec 24;14(1):11. doi: 10.3390/ijerph14010011.
Abstract. In this study, we developed curcumin-encapsulated hyaluronic acid-polylactide nanoparticles (CEHPNPs) to be used for liver fibrosis amelioration. CD44, the hyaluronic acid (HA) receptor, is upregulated on the surface of cancer cells and on activated hepatic stellate cells (aHSCs) rather than normal cells. CEHPNPs could bind to CD44 and be internalized effectively through endocytosis to release curcumin, a poor water-soluble liver protective agent. Thus, CEHPNPs were potentially not only improving drug efficiency, but also targeting aHSCs. HA and polylactide (PLA) were crosslinked by adipic acid dihydrazide (ADH). The synthesis of HA-PLA was monitored by Fourier-transform infrared (FTIR) and Nuclear Magnetic Resonance (NMR). The average particle size was approximately 60-70 nm as determined by dynamic light scattering (DLS) and scanning electron microscope (SEM). Zeta potential was around -30 mV, which suggested a good stability of the particles. This drug delivery system induced significant aHSC cell death without affecting quiescent HSCs, hepatic epithelial, and parenchymal cells. This system reduced drug dosage without sacrificing therapeutic efficacy. The cytotoxicity IC50 (inhibitory concentration at 50%) value of CEHPNPs was approximately 1/30 to that of the free drug treated group in vitro. Additionally, the therapeutic effects of CEHPNPs were as effective as the group treated with the same curcumin dose intensity in vivo. CEHPNPs significantly reduced serum aspartate transaminase/alanine transaminase (ALT/AST) significantly, and attenuated tissue collagen production and cell proliferation as revealed by liver biopsy. Conclusively, the advantages of superior biosafety and satisfactory therapeutic effect mean that CEHPNPs hold great potential for treating hepatic fibrosis.
(3) Zheng R, Deng Q, Liu Y, Zhao P. Curcumin Inhibits Gastric Carcinoma Cell Growth and Induces Apoptosis by Suppressing the Wnt/β-Catenin Signaling Pathway. Med Sci Monit. 2017 Jan 12;23:163-171. doi: 10.12659/msm.902711.
Abstract. BACKGROUND Curcumin has well-known, explicit biological anti-tumor properties. The Wnt/β-catenin signaling pathway plays a central role in tumor cell proliferation and curcumin can regulate the Wnt/b-catenin signaling pathway of several carcinomas. The aim of this study was to investigate the impact of curcumin on the Wnt/β-catenin signaling pathway in human gastric cancer cells. MATERIAL AND METHODS We used 3 gastric cancer cell lines: SNU-1, SNU-5, and AGS. Research methods used were MTT assay, flow cytometry, clonogenic assay, annexin V/PI method, Western blotting analysis, tumor formation assay, and in vivo in the TUNEL assay. RESULTS Curcumin markedly impaired tumor cell viability and induced apoptosis in vitro. Curcumin significantly suppressed the levels of Wnt3a, LRP6, phospho-LRP6, β-catenin, phospho-β-catenin, C-myc, and survivin. Xenograft growth in vivo was inhibited and the target genes of Wnt/β-catenin signaling were also reduced by curcumin treatment. CONCLUSIONS Curcumin exerts anti-proliferative and pro-apoptotic effect in gastric cancer cells and in a xenograft model. Inhibition of the Wnt/β-catenin signaling pathway and the subsequently reduced expression of Wnt target genes show potential as a newly-identified molecular mechanism of curcumin treatment.
Zhang N, Gao M, Wang Z, Zhang J, Cui W, Li J, Zhu X, Zhang H, Yang DH, Xu X. Curcumin reverses doxorubicin resistance in colon cancer cells at the metabolic level. J Pharm Biomed Anal. 2021 May 7;201:114129. doi: 10.1016/j.jpba.2021.114129.
(4) Santos-Parker JR, Strahler TR, Bassett CJ, Bispham NZ, Chonchol MB, Seals DR. Curcumin supplementation improves vascular endothelial function in healthy middle-aged and older adults by increasing nitric oxide bioavailability and reducing oxidative stress. Aging (Albany NY). 2017 Jan 3;9(1):187-208. doi: 10.18632/aging.101149.
Abstract. We hypothesized that curcumin would improve resistance and conduit artery endothelial function and large elastic artery stiffness in healthy middle-aged and older adults. Thirty-nine healthy men and postmenopausal women (45-74 yrs) were randomized to 12 weeks of curcumin (2000 mg/day Longvida®; n=20) or placebo (n=19) supplementation. Forearm blood flow response to acetylcholine infusions (FBFACh; resistance artery endothelial function) increased 37% following curcumin supplementation (107±13 vs. 84±11 AUC at baseline, P=0.03), but not placebo (P=0.2). Curcumin treatment augmented the acute reduction in FBFACh induced by the nitric oxide synthase inhibitor NG monomethyl-L-arginine (L-NMMA; P=0.03), and reduced the acute increase in FBFACh to the antioxidant vitamin C (P=0.02), whereas placebo had no effect (both P>0.6). Similarly, brachial artery flow-mediated dilation (conduit artery endothelial function) increased 36% in the curcumin group (5.7±0.4 vs. 4.4±0.4% at baseline, P=0.001), with no change in placebo (P=0.1). Neither curcumin nor placebo influenced large elastic artery stiffness (aortic pulse wave velocity or carotid artery compliance) or circulating biomarkers of oxidative stress and inflammation (all P>0.1). In healthy middle-aged and older adults, 12 weeks of curcumin supplementation improves resistance artery endothelial function by increasing vascular nitric oxide bioavailability and reducing oxidative stress, while also improving conduit artery endothelial function.
(5) de Alcântara GF, Simões-Neto E, da Cruz GM, Nobre ME, Neves KR, de Andrade GM, Brito GA, Viana GS. Curcumin reverses neurochemical, histological and immuno-histochemical alterations in the model of global brain ischemia. J Tradit Complement Med. 2016 Feb 11;7(1):14-23. doi: 10.1016/j.jtcme.2015.10.001.
Abstract. Curcumin, a curcuminoid from Curcuma longa, presents antioxidant and anti-inflammatory actions and, among pathological changes of cerebral ischemic injury, inflammation is an important one. The objectives were to study the neuroprotective action of curcumin, in a model of global ischemia. Male Wistar rats (sham-operated, ischemic untreated and ischemic treated with curcumin, 25 or 50 mg/kg, p.o.) were anesthesized and their carotid arteries occluded, for 30 min. The SO group had the same procedure, except for carotid occlusion. In the 1st protocol, animals were treated 1 h before ischemia and 24 h later; and in the 2nd protocol, treatments began 1 h before ischemia, continuing for 7 days. Twenty four hours after the last administration, animals were euthanized and measurements for striatal monoamines were performed, at the 1st and 7th days after ischemia, as well as histological and immunohistochemical assays in hippocampi. We showed in both protocols, depletions of DA and its metabolites (DOPAC and HVA), in the ischemic group, but these effects were reversed by curcumin. Additionally, a decrease seen in 5-HT contents, 1 day after ischemia, was also reversed by curcumin. This reversion was not seen 7 days later. On the other hand, a decrease observed in NE levels, at the 7th day, was totally reversed by curcumin. Furthermore, curcumin treatments increased neuronal viability and attenuated the immunoreactivity for COX-2 and TNF-alpha, in the hippocampus in both protocols. We showed that curcumin exerts neuroprotective actions, in a model of brain ischemia that are probably related to its anti-inflammatory activity.
(6) Zhang H, Zhao W, Malhotra A. Efficacy of Curcumin in Ameliorating Aluminum- Induced Neurotoxicity. J Environ Pathol Toxicol Oncol. 2018;37(2):163-172. doi: 10.1615/JEnvironPatholToxicolOncol.2018026437.
Abstract. Background-The present study evaluated the efficacy of curcumin as a nutritional supplement in preventing aluminum-induced neurotoxicity in rats. Methods-The rats were segregated into four groups, which included normal controls and aluminum-treated, curcumin- treated, and aluminum- and curcumin-treated animals. Results-Eight weeks of aluminum treatment resulted in a significant increase in the levels of lipid peroxidation (LPO) and reactive oxygen species (ROS) in both cerebellum and cerebrum as compared to normal animals. In contrast, the activities of glutathione-S-transferase (GST), glutathione reductase (GR), superoxide dismutase (SOD), and reduced glutathione (GSH) levels were found to be significantly decreased following aluminum treatment. Furthermore, aluminum resulted in anxiety in rats as determined with the elevated plus maze test. In addition, an appreciable decrease was noticed in both muscular and locomotor activity of aluminum-treated animals, as determined by rotarod and actophotometer tests, respectively. However, in aluminum-treated animals that also received curcumin supplements, the already raised levels of LPO and ROS returned to near normal limits in the cerebrum. Moreover, curcumin treatment of the aluminum-treated animals also resulted in a significant improvement in the levels of GSH and enzyme activities of GST in both the cerebrum and cerebellum. Also, improvement was observed in the behavior of aluminum-treated animals upon curcumin supplementation. Conclusion-The present study suggests that curcumin may act as a neuroprotectant against aluminum-induced neurodegenerative and behavioral disorders, but further investigations are needed to understand the exact mechanism of neuroprotection.
| Evaluate |

