| "Descrizione" by admin (19549 pt) | 2024-May-29 16:27 |
Indigo (CI 73000), 2-(1,3-Dihydro-3-oxo-2H-indazol-2-ylidene)-1,2-dihydro-3H-indol-3-one is known as Indigo Blue, an artificial dye used mainly in the textile industry and in the coloring of cosmetic products.
Indigo is a synthetic organic dye belonging to the indigoid family of dyes, structurally similar to natural indigo but obtained through chemical synthesis. The molecule is characterized by the presence of two indole nuclei linked by an indazole bridge, which imparts stability and color intensity to the compound. Its primary use as a pigment is due to its ability to produce a wide range of deep blue shades, highly sought after in various industrial and cosmetic applications.
Chemical Industrial Synthesis Process
- Preparation of reagents. The main raw materials include aniline, phthalonitrile, hydrochloric acid (HCl), sodium chloride (NaCl), sodium hydroxide (NaOH), and organic solvents such as ethanol or acetone.
- Synthesis of indazole. The production of Indigo begins with the synthesis of indazole. Aniline is treated with phthalonitrile in the presence of hydrochloric acid and sodium chloride to form an intermediate.
- Cyclization. The intermediate is cyclized to form indazole. This reaction is carried out under high-temperature and controlled pressure conditions.
- Synthesis of indolone. The indazole is further treated with sodium hydroxide and an organic solvent to form indolone. This reaction occurs in an alkaline environment.
- Condensation. The indazole and indolone are reacted in a condensation reaction to form the indigoid chromophore, 2-(1,3-Dihydro-3-oxo-2H-indazol-2-ylidene)-1,2-dihydro-3H-indol-3-one.
- Purification. The crude product is purified using techniques such as crystallization, filtration, and chromatography to remove impurities and achieve a high-purity colorant.
- Stabilization. The purified Indigo is stabilized to ensure its stability during transportation and storage, preventing degradation and oxidation.
- Drying. The purified product is dried at controlled temperatures to obtain a fine powder.
- Quality control. The Indigo undergoes rigorous quality testing to ensure it meets standards for purity, color intensity, and safety. These tests include chemical analysis, spectroscopy, and microbiological testing.
What it is used for and where
Cosmetics
Restricted cosmetic ingredient as IV/98 a Relevant Item in the Annexes of the European Cosmetics Regulation 1223/2009. Substance or ingredient reported:
- 2-(1,3-Dihydro-3-oxo-2H-indazol-2-ylidene)-1,2-dihydro-3H-indol-3-one
In cosmetics, Indigo is used to impart color to products such as eyeshadows, eyeliner pencils, and other makeup products where intense and lasting blue coloration is required. Its thermal and light stability makes it ideal for such applications, ensuring uniform and long-lasting coloration. It is also used in some formulations of temporary tattoo inks and other artistic products.
Medical
Indigoid dyes are widely used as well as in the textile and cosmetic industry, in medicines as astringents for the treatment of infections, ulcers, gastroenteritis, however these dyes have proven genotoxic at high concentrations (1).
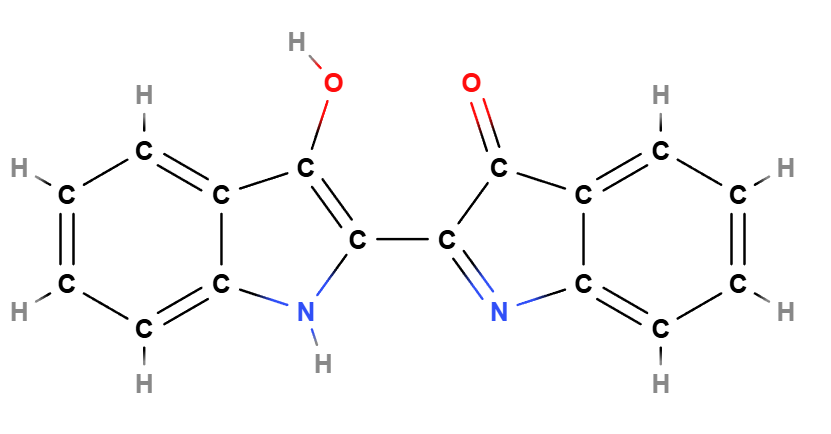 |  |
Molecular Formula C16H10N2O2
Molecular Weight 262.26 g/mol
CAS 482-89-3
UNII 1G5BK41P4F
EC Number 207-586-9
Synonyms:
indigo
Indigo Blue
Bibliografia_____________________________________________________________________
(1) Karsli-Ceppioglu, S., & Yurdun, T. (2012). In vitro testing for genotoxicity of indigoid dyes by comet assay. Clinical and Experimental Health Sciences, 2(3), 108.
Abstract. Indigo and indigoid dyes are natural dyes and have been known since Bronze Age (B.C. 2000) (1,2). Most natural indigoid dyes were obtained from woad (Isatis tinctoria L., Brassicaceae, also known as dyer’s woad) and the indigo plant (Indigofera tinctoria L.) in temperate climates. Isatis tinctoria L. leaf and root extracts were shown in vitro and in vivo studies to be antibacterial and antiviral. Although indigo-related pigments are widely used, there are few studies about its genotoxic effects which show mutagenicity on Salmonella typhimurium. On the other hand, to evaluate the hazardous effects of dyes on DNA is very important to protect industry workers, who are exposed to dyes and the environment. Alkaline single-cell gel electrophoresis (SCGE-Comet assay) is a sensitive genotoxicity assessment, which detects DNA-strand breaks and alkali-labile sites within a relatively short period of time and cost-effective manner. The comet assay has been used for the assessment of genetic damage in vitro and in vivo in a great variety of cells (5,6). It is used to assess the genotoxicity of industrial chemicals (new chemicals and existing chemicals) as well as for agrochemicals, biocides, pharmaceuticals and physical agents (7). The aim of this study was to estimate DNA damage of indigotin, 6-bromo indigotin, indirubin and 6-bromo indirubin by in vitro alkaline comet assay in the peripheral lymphocytes.
| Evaluate |

