Check the ingredients!
... live healthy!


| "Descrizione" by Al222 (19776 pt) | 2024-Jun-20 09:18 |
Ethyl Oleate is a chemical compound, an ester of ethanol and oleic acid, commonly used in various industrial, pharmaceutical, and cosmetic applications. It is valued for its excellent solubilizing properties, skin conditioning abilities, and as a medium for drug delivery.
Chemical Composition and Structure
Ethyl Oleate is an ester formed from the reaction of ethanol and oleic acid, a monounsaturated fatty acid found in vegetable oils. The chemical formula for Ethyl Oleate is C20H38O2. The chemical structure consists of an oleic acid chain esterified with an ethyl group, giving it lipophilic properties and good solubility in oils and organic solvents.
Physical Properties
Ethyl Oleate typically appears as a clear, colorless to slightly yellowish liquid. It is soluble in oils and organic solvents but insoluble in water. It is known for its low viscosity and ability to easily penetrate the skin, making it a valuable ingredient in topical formulations.
Production Process
The production of Ethyl Oleate involves the esterification reaction between oleic acid and ethanol. The process requires controlled temperature conditions and the presence of an acid catalyst to facilitate the formation of the ester. The final product is purified to remove any impurities, ensuring high quality.
Chemical Industrial Synthesis Process
What it is used for and where
Cosmetics - INCI Functions
Skin conditioning agent - Emollient. Emollients have the characteristic of enhancing the skin barrier through a source of exogenous lipids that adhere to the skin, improving barrier properties by filling gaps in intercorneocyte clusters to improve hydration while protecting against inflammation. In practice, they have the ability to create a barrier that prevents transepidermal water loss. Emollients are described as degreasing or refreshing additives that improve the lipid content of the upper layers of the skin by preventing degreasing and drying of the skin. The problem with emollients is that many have a strong lipophilic character and are identified as occlusive ingredients; they are oily and fatty materials that remain on the skin surface and reduce transepidermal water loss. In cosmetics, emollients and moisturisers are often considered synonymous with humectants and occlusives.
Perfuming. Unlike fragrance, which can also contain slightly less pleasant or characteristic odours, the term perfume indicates only very pleasant fragrances. Used for perfumes and aromatic raw materials.
Industrial Applications
Drug Delivery Vehicle: Ethyl Oleate is used as a vehicle for drug delivery, particularly in injectable formulations. Its ability to dissolve a wide range of medicinal compounds and penetrate tissues makes it ideal for this purpose (1).
Skin Care: In skin care products, Ethyl Oleate acts as an emollient and conditioning agent, improving skin elasticity and hydration. It is commonly used in lotions, creams, and massage oils for its ability to enhance product texture and facilitate absorption.
Hair Care: It is also present in some hair care products, such as oils and conditioning treatments, where it helps nourish and soften the hair without leaving it greasy.
Food Industry: Although primarily used in cosmetics and pharmaceuticals, Ethyl Oleate can be used as a food additive (2), mainly as a coating agent or a component in food-grade lubricants.
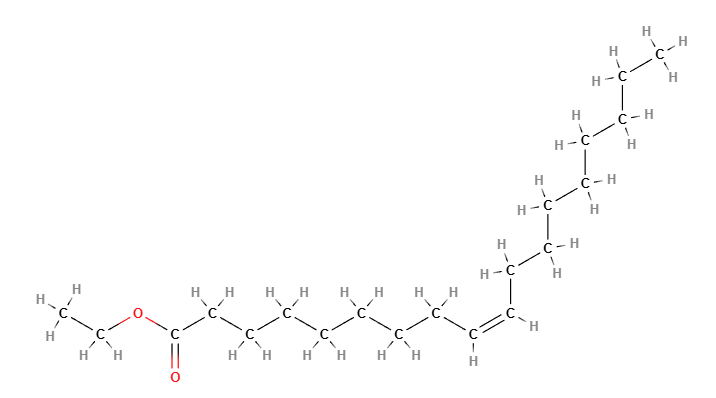 |  |
Molecular Formula C20H38O2
Molecular Weight 310.5 g/mol
CAS 111-62-6
UNII Z2Z439864Y
EC Number 203-889-5
Synonyms:
Ethyl cis-9-octadecenoate
References__________________________________________________________________________
(1) Sha K, Ma Q, Veroniaina H, Qi X, Qin J, Wu Z. Formulation optimization of solid self-microemulsifying pellets for enhanced oral bioavailability of curcumin. Pharm Dev Technol. 2021 Jun;26(5):549-558. doi: 10.1080/10837450.2021.1899203.
Abstract. Solidification of self-microemulsifying drug delivery systems (SMEDDS) is one of the major trends to promote the transformation of self-microemulsion technology into industrialization. Here, a preliminary curcumin SMEDDS formulation was constructed to improve the druggability of curcumin, through the determination of equilibrium solubility determination, self-emulsifying grading assessment, and pseudo-ternary phase diagrams drafting. Furthermore, the optimal curcumin SMEDDS formulation consisted of 10% Ethyl oleate, 57.82% Cremophor RH 40, and 32.18% Transcutol P was obtained by the simplex lattice design. Besides, curcumin solid self-microemulsifying drug delivery system (S-SMEDDS) was developed by the extrusion and spheronization process to achieve the solidification of SMEDDS. The formulation of curcumin S-SMEDDS pellets was screened by the single factor experiment and the process parameters were investigated using the orthogonal optimization method. Subsequently, curcumin S-SMEDDS pellets were evaluated by apparent morphology characterization, redispersibility study, drug release behavior, and pharmacokinetic evaluation. Results from the pharmacokinetic study in rabbits showed that the AUC0-τ of the curcumin S-SMEDDS pellets and curcumin suspension were 5.91 ± 0.28 µg/mL·h and 2.05 ± 0.04 µg/mL·h, while the relative bioavailability was 289.30%. These studies demonstrated that S-SMEDDS pellets can be a promising strategy for curcumin industrialized outputs.
Zhang Y, Li Z, Zhang K, Yang G, Wang Z, Zhao J, Hu R, Feng N. Ethyl oleate-containing nanostructured lipid carriers improve oral bioavailability of trans-ferulic acid ascompared with conventional solid lipid nanoparticles. Int J Pharm. 2016 Sep 10;511(1):57-64. doi: 10.1016/j.ijpharm.2016.06.131.
(2) Kemp CJ, D'Alessio DA, Scott RO, Kelm GR, Meller ST, Barrera JG, Seeley RJ, Clegg DJ, Benoit SC. Voluntary consumption of ethyl oleate reduces food intake and body weight in rats. Physiol Behav. 2008 Mar 18;93(4-5):912-8. doi: 10.1016/j.physbeh.2007.12.008.
Abstract. Previous studies have shown that administration of the fatty acids, linoleic and oleic acid, either by intragastric or intraintestinal infusion, suppresses food intake and body weight in rats. While still not fully understood, gut-mediated satiety mechanisms likely are potential effectors of this robust response to gastrointestinal fatty acid infusions. The objective of this study was to assess the effects of voluntary access to an oleic acid derivative, ethyl oleate (EO), on subsequent food intake and body weight in rats. Animals were randomized either to a 12.5% EO diet or a soybean oil diet as a "breakfast," followed either by two one-hour or one five-hour access periods to standard rodent diet, and food intake and body weights were collected. Across 14 days access, rats consuming EO on both feeding schedules gained less weight and consumed less total kilocalories than rats consuming the SO diet. Further, plasma levels of glucose and insulin were comparable in both EO and SO diet groups. In summary, EO was found to increase weight loss in rats maintained on a 75% food-restriction regimen, and attenuate weight-gain upon resumption of an ad-libitum feeding regimen. These data indicate that voluntary access to EO promoted short-term satiety, compared to SO diet, and that these effects contributed to an important and novel attenuated weight gain in EO-fed animals.
| Evaluate |