Check the ingredients!
... live healthy!


| "Descrizione" by admin (19362 pt) | 2024-Oct-06 10:50 |
Solvent Yellow 3, also known as 2-Aminoazotoluene, is a synthetic azo dye with the chemical formula C14H15N3. It is widely used in industrial applications such as coloring plastics, oils, waxes, and, historically, textiles and consumer products. However, its use has been heavily restricted due to concerns about its classification as a potential human carcinogen. The International Agency for Research on Cancer (IARC) classifies Solvent Yellow 3 as a Group 2B carcinogen, "possibly carcinogenic to humans," and the European Union lists it in Category 1B, indicating a high risk of carcinogenicity. The U.S. National Toxicology Program (NTP) classifies it as "reasonably anticipated to be a human carcinogen." A significant concern is its ability to release aromatic amines, such as o-toluidine, through the cleavage of the azo bond, which is also classified as a carcinogen (EU22).
Chemical Composition and Structure
Solvent Yellow 3 is an azo dye composed of an azo bond (-N=N-) connecting an amino group (-NH2) and a toluene group (C6H5-CH3).The azo bond gives the dye its characteristic bright yellow-orange color. Its chemical structure makes it lipophilic, meaning it is soluble in organic solvents such as oils and hydrocarbons but insoluble in water. The primary health concern with this structure is its potential to degrade, releasing harmful aromatic amines such as o-toluidine, a known carcinogen listed as an EU22 substance.
Physical Properties
Solvent Yellow 3 typically appears as a yellow-orange crystalline powder or solid. It is insoluble in water but highly soluble in organic solvents like oils, alcohols, and hydrocarbons, making it suitable for applications in products such as plastics, waxes, and lubricants. It has good thermal and light stability, making it useful for applications where resistance to environmental conditions is necessary.
Production Process
Solvent Yellow 3 is produced through an azo coupling reaction, where aromatic amines, such as o-toluidine, are reacted with nitrous acid to form a diazonium salt, which is then coupled with another aromatic compound. The process must be strictly controlled to ensure the purity and stability of the dye due to the potential hazards associated with its components and by-products.
Applications
Industrial Use: Solvent Yellow 3 is primarily used to color:
Historical Use in Consumer Products: In the past, Solvent Yellow 3 was used in cosmetics, textile dyes, and other consumer products. However, due to its carcinogenic classification, its use in these sectors has been significantly reduced or banned.
Health and Safety Concerns
Carcinogenicity
Solvent Yellow 3 is classified by the IARC as a Group 2B carcinogen, meaning it is "possibly carcinogenic to humans." The European Union lists it as a Category 1B carcinogen, indicating a high risk of causing cancer, based on strong evidence of its potential to cause cancer in humans. The primary concern is its ability to release aromatic amines, such as o-toluidine, due to azo bond cleavage. The NTP in the U.S. also classifies it as "reasonably anticipated to be a human carcinogen." o-Toluidine is a known carcinogen, linked particularly to bladder cancer.

Toxicity
Beyond its carcinogenic potential, Solvent Yellow 3 can cause acute toxicity if ingested, inhaled, or absorbed through the skin. Prolonged exposure may lead to skin irritation, respiratory issues, and an increased risk of cancer. Because of its ability to degrade into hazardous compounds like o-toluidine, handling and use of this dye must be done with extreme care, particularly in industrial settings.
Aromatic Amines EU22
One of the greatest risks associated with Solvent Yellow 3 is the release of aromatic amines, such as o-toluidine, classified in the EU22 list of hazardous substances. The cleavage of the azo bond can lead to the formation of these amines, which are known carcinogens and pose significant health risks, particularly for bladder cancer. EU regulations strictly control the use and handling of these substances to limit human exposure.
Environmental and Safety Considerations
Solvent Yellow 3 is not biodegradable and can persist in the environment, particularly in aquatic ecosystems. Its toxicity and ability to release aromatic amines make it a hazardous substance for the environment. Its use requires strict waste management protocols to prevent contamination. Environmental regulations, particularly in the European Union and the U.S., mandate that industries using this dye follow stringent guidelines for waste disposal and worker safety.
Regulatory Status
Due to its classification as a potential carcinogen and the risk of releasing hazardous aromatic amines, Solvent Yellow 3 is heavily regulated. In the European Union, it is classified as a Category 1B carcinogen, and its use in consumer products is severely restricted. In the United States, the NTP classifies it as "reasonably anticipated to be a human carcinogen," and the FDA and EPA have limited its use. In many countries, Solvent Yellow 3 is banned or heavily regulated in cosmetics and textiles.
Safety
The problem with azo dyes (monoazo or diazo) is photocatalytic degradation leading to oxidation and the subsequent formation of impurities such as aromatic amines, some of which have carcinogenic activity. (Chung KT, Stevens SE Jr, Cerniglia CE. The reduction of azo dyes by the intestinal microflora. Crit Rev Microbiol. 1992;18(3):175-90. doi: 10.3109/10408419209114557. )
The study cited in Bibliography finds that: Overall, Solvent Yellow 3 exhibited carcinogenicity and genotoxicity in experimental animals. It induced gene mutations in vivo and in vitro. It showed clastogenicity in vivo and to a lesser extent in vitro. It caused DNA damages in vivo and in vitro. Following oral administration, Solvent Yellow 3 induced liver tumours in mice, rats, hamsters and dogs, induced urinary bladder tumours and gall bladder tumours in hamsters and dogs and induced lung hemangioendotheliomas in mice. It also induced liver tumours in mice via dermal exposure. Evidence from a wide range of in vivo and in vitro genotoxicity assays indicates a potent genotoxicity of Solvent Yellow 3, which may have played a role in Solvent Yellow 3–induced tumor formation.
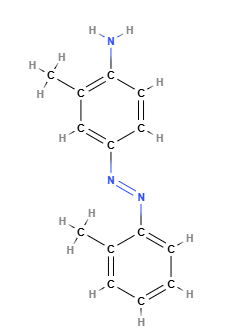 |  |
Molecular Formula C14H15N3
Molecular Weight 225.29 g/mol
CAS 97-56-3
UNII QHZ900P7ZA
EC Number 202-591-2
DTXSID1020069
CHEMBL83552
Synonyms:
2-Aminoazotoluene
o-Aminoazotoluene
Fast Garnet GBC Base
Solvent Yellow 3
References__________________________________________________________________________
Screening Assessment Aromatic Azo and Benzidine-based Substance GroupingCertain Azo Solvent Dyes. Environment and Climate Change Canada Health Canada May 2016
Synopsis. Pursuant to sections 68 or 74 of the Canadian Environmental Protection Act, 1999 (CEPA 1999), the Ministers of the Environment and of Health have conducted a screening assessment on 22 Azo Solvent Dyes. These substances constitute a subgroup of the Aromatic Azo and Benzidine-based Substance Grouping being assessed as part of the Groupings Initiative of Canada’s Chemicals Management Plan (CMP) based on structural similarity and applications. Substances in this grouping were identified as priorities for assessment as they met the categorization criteria under subsection 73(1) of CEPA 1999 and/or were considered as a priority based on other human health concerns. The Chemical Abstracts Service Registry Number (CAS RN)1, Domestic Substances List (DSL) name, and Colour Index (C.I) name or common name of the 22 substances are presented in the following table.....
| Evaluate |