| "Descrizione" by Al222 (20718 pt) | 2024-Oct-21 11:35 |
Isopropanolamine is an organic chemical compound that belongs to the class of alkanolamines, which are amines containing a hydroxyl group (-OH). This hybrid structure gives isopropanolamine unique properties, making it useful across various industrial applications. It is widely employed as a pH regulator, emulsifier, and chemical intermediate in industries such as cosmetics and detergents. Its ability to interact with both water and oils enhances its effectiveness in formulations that require chemical stability. Typically, it appears as a clear, colorless liquid, sometimes with a faint yellow tint, and has a mild ammonia-like odor.
Chemical Composition and Structure
The chemical formula of Isopropanolamine is C3H9NO. Its molecular structure comprises an isopropyl group (CH3-CH(OH)-CH3) attached to an amine group (-NH2) and a hydroxyl group (-OH), providing it with both basic and hydrophilic properties. The presence of the amine group makes it a good proton acceptor, explaining its effectiveness as a pH regulator. The hydroxyl group improves the molecule's solubility in water and polar solvents. Isopropanolamine exists in various forms depending on the number of amine or hydroxyl groups attached, resulting in different variants like monoisopropanolamine (MIPA), diisopropanolamine (DIPA), and triisopropanolamine (TIPA), each having specialized applications.
Physical Properties
It is a viscous, colorless liquid with a boiling point of about 160°C. It is fully miscible with water and a wide range of organic solvents, such as alcohols, ketones, and glycols. Its pH typically ranges from 9 to 11, making it a suitable buffering agent for applications requiring stable pH levels. Its density is approximately 0.96 g/cm³ at 25°C. Isopropanolamine is relatively stable but can react with strong oxidizers.

Production Process
Isopropanolamine is industrially produced through an alkylation reaction between propylene oxide (C3H6O) and ammonia (NH3). This process yields various alkanolamines, with Isopropanolamine being one of the key products. The reaction can be adjusted to produce different ratios of mono-, di-, and triisopropanolamine, with the resulting by-products separated via fractional distillation. The purity of the produced Isopropanolamine is crucial for its use in cosmetics and other sensitive applications, where impurities could negatively affect the final product’s stability.
Applications
Cosmetics: Isopropanolamine serves as buffer and a pH regulator in cosmetic formulations, ensuring that products maintain long-term chemical stability. It is also used as an emulsifier, helping to keep mixtures of oils and water stable. Commonly found in lotions, moisturizers, facial cleansers, shampoos, and conditioners, its buffering capacity is essential in products that must maintain a specific pH to avoid skin irritation.
Detergents and Cleaning Products: Isopropanolamine is widely used in industrial and household cleaners for its ability to neutralize fatty acids and stabilize pH, while enhancing cleaning effectiveness. It is also used in liquid soaps and metal cleaning products.
INCI Functions:
Buffering agent. It is an iingredient that can bring an alkaline or acid solution to a certain pH level and prevent it from changing, in practice a pH stabiliser that can effectively resist instability and pH change.
Cosmetic safety
Restricted cosmetic ingredient as III/61 a Relevant Item in the Annexes of the European Cosmetics Regulation 1223/2009. Substance or ingredient reported: Monoalkylamines, monoalkanolamines and their salts.
Maximum concentration in ready for use preparation: Maximum secondary amine content: 0.5%. Other - Do not use with nitrosating systems - Minimum purity: 99% - Maximum secondary amine content: 0.5% (applies to raw materials) - Maximum nitrosamine content: 50 microgram/kg - Keep in nitrite-free containers
Environmental and Safety Considerations
Isopropanolamine is generally regarded as safe for topical use in cosmetics and detergents, provided it is used according to established guidelines. However, as with all chemicals, it is important to follow proper guidelines to avoid skin irritations or allergic reactions, especially for individuals with sensitive skin. Its irritant properties increase at higher concentrations, making it crucial to adhere to recommended dosages in consumer products. Environmentally, Isopropanolamine is biodegradable and does not tend to accumulate in ecosystems. However, sustainable production practices and proper disposal methods are necessary to prevent large-scale environmental contamination.
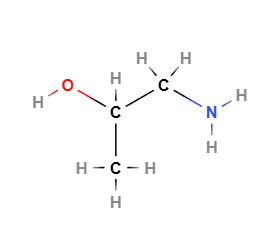 |  |
Molecular Formula C3H9NO
Molecular Weight 75.11 g/mol
CAS 78-96-6
UNII UE40BY1BZW
EC Number 201-162-7
CHEBI:19030
CHEMBL326602
FEMA 3965
DTXSID9021764
Nikkaji J1.961E J2.150D
Synonyms:
1-Aminopropan-2-ol
Amino-2-propanol
References__________________________________________________________________________
Writer, C. I. R. (2024). Safety Assessment of Diisopropanolamine, Triisopropanolamine, Isopropanolamine, and Mixed lsopropanolamines as Used in Cosmetics. The Expert Panel for Cosmetic Ingredient Safety members are: Chair, Wilma F. Bergfeld, M.D., F.A.C.P.; Donald V. Belsito, M.D.; David E. Cohen, M.D.; Curtis D. Klaassen, Ph.D.; Allan E. Rettie, Ph.D.; David Ross, Ph.D.; Thomas J. Slaga, Ph.D.;Paul W. Snyder, D.V.M., Ph.D.; and Susan C. Tilton, Ph.D. The Cosmetic Ingredient Review (CIR) Executive Director is Bart Heldreth, Ph.D., and the Senior Director is Monice Fiume, M.B.A. This safety assessment was prepared by Preethi Raj, M.Sc., Senior Scientific Analyst/Writer, CIR.
Feng YM, Qi PY, Xiao WL, Zhang TH, Zhou X, Liu LW, Yang S. Fabrication of Isopropanolamine-Decorated Coumarin Derivatives as Novel Quorum Sensing Inhibitors to Suppress Plant Bacterial Disease. J Agric Food Chem. 2022 May 25;70(20):6037-6049. doi: 10.1021/acs.jafc.2c01141.
Abstract. Emerging pesticide-resistant phytopathogenic bacteria have become a stumbling block in the development and use of pesticides. Quorum sensing (QS) blockers, which interfere with bacterial virulence gene expression, are a compelling way to manage plant bacterial disease without resistance. Herein, a series of isopropanolamine-decorated coumarin derivatives were designed and synthesized, and their potency in interfering with QS was investigated. Notably, compound A5 exhibited a better bioactivity with median effective concentration (EC50) values of 6.75 mg L-1 against Xanthomonas oryzae pv. oryzae (Xoo) than bismerthiazol (EC50 = 21.9 mg L-1). Further biochemical studies revealed that compound A5 disturbed biofilm formation and suppressed bacterial virulence factors and so forth. Moreover, compound A5 decreased the expression of QS-related genes. Interestingly, compound A5 had the acceptable control effect (53.2%) toward Xoo in vivo. Overall, this study identifies a novel lead compound for the development of bactericide candidates to control plant bacterial diseases by interfering with QS.
Li Z, Yang B, Liu H, Ding Y, Fang Z, Shao W, Qi P, Zhou X, Liu L, Yang S. The Discovery of Novel Ferulic Acid Derivatives Incorporating Substituted Isopropanolamine Moieties as Potential Tobacco Mosaic Virus Helicase Inhibitors. Int J Mol Sci. 2022 Nov 13;23(22):13991. doi: 10.3390/ijms232213991.
Abstract. Target-based drug design, a high-efficiency strategy used to guide the development of novel pesticide candidates, has attracted widespread attention. Herein, various natural-derived ferulic acid derivatives incorporating substituted isopropanolamine moieties were designed to target the tobacco mosaic virus (TMV) helicase. Bioassays demonstrating the optimized A19, A20, A29, and A31 displayed excellent in vivo antiviral curative abilities, affording corresponding EC50 values of 251.1, 336.2, 347.1, and 385.5 μg/mL, which visibly surpassed those of commercial ribavirin (655.0 μg/mL). Moreover, configurational analysis shows that the R-forms of target compounds were more beneficial to aggrandize antiviral profiles. Mechanism studies indicate that R-A19 had a strong affinity (Kd = 5.4 μM) to the TMV helicase and inhibited its ability to hydrolyze ATP (50.61% at 200 μM). Meanwhile, A19 could down-regulate the expression of the TMV helicase gene in the host to attenuate viral replication. These results illustrate the excellent inhibitory activity of A19 towards the TMV helicase. Additionally, docking simulations uncovered that R-A19 formed more hydrogen bonds with the TMV helicase in the binding pocket. Recent studies have unambiguously manifested that these designed derivatives could be considered as promising potential helicase-based inhibitors for plant disease
Zhao YL, Huang X, Liu LW, Wang PY, Long QS, Tao QQ, Li Z, Yang S. Identification of Racemic and Chiral Carbazole Derivatives Containing an Isopropanolamine Linker as Prospective Surrogates against Plant Pathogenic Bacteria: In Vitro and In Vivo Assays and Quantitative Proteomics. J Agric Food Chem. 2019 Jul 3;67(26):7512-7525. doi: 10.1021/acs.jafc.9b02036.
Abstract. Recent observations on the emergence of drug-resistant plant pathogenic bacteria have highlighted and elicited an acute campaign to develop novel, highly efficient antibiotic surrogates for managing bacterial diseases in agriculture. Thus, a type of racemic and chiral carbazole derivative containing an isopropanolamine pattern was systematically synthesized to discover low-cost and efficient antibacterial candidates. Screening results showed that compounds 2f, 6c, and 2j could significantly suppress the growth of tested plant pathogens, namely Xanthomonas oryzae pv oryzae, X. axonopodis pv citri, and Pseudomonas syringae pv actinidiae, and provided the corresponding EC50 values of 1.27, 0.993, and 0.603 μg/mL, which were significantly better than those of existing commercial drugs. In vivo studies confirmed their prospective applications for controlling plant bacterial diseases. Label-free quantitative proteomics analysis indicated that compound 2f could dramatically induce the up- and down-regulation of a total of 247 differentially expressed proteins, which was further validated by the parallel reaction monitoring technique. Moreover, fluorescence spectra and SEM images were obtained to further explore the antibacterial mechanism.
| Evaluate |

