| "Descrizione" by Al222 (20718 pt) | 2024-Oct-21 17:10 |
Basic Blue 99 is a dye from the naphthoquinoneimine class, primarily used in cosmetic and hair care products such as permanent and semi-permanent hair dyes. It is appreciated for its intense blue hue and ability to bind effectively to hair fibers and other substrates. Known for its color stability and longevity, it is commonly used in combination with other dyes to create a wide range of shades. Basic Blue 99 typically appears as a powder or solution with a dark blue or black-blue color.
Chemical Composition and Structure
The chemical structure of Basic Blue 99 is based on a naphthoquinone system, an aromatic compound containing two ketone groups (C=O) attached to a naphthalene ring, along with an amine (imine) component that contributes to its coloration. The molecule may contain various functional groups and substituents that affect its solubility and ability to interact with hair fibers and other substrates. This dye is water-soluble and can chemically bond to keratin proteins, making it effective for permanent hair coloring.
Physical Properties
It appears as a solid powder or granules, soluble in water. The color varies from intense blue to dark blue, depending on concentration and solvent used. Its water solubility allows it to penetrate hair fibers effectively, providing even coloration. At high temperatures, the dye may decompose, releasing by-products that can affect color stability. It exhibits good resistance to light and oxidation, which contributes to its long-lasting color retention.
Production Process
Basic Blue 99 is produced through a series of chemical reactions involving aromatic intermediates, such as naphthoquinone derivatives and amine groups. The process involves a substitution reaction on an aromatic ring, forming an imine bond. The synthesis may include multiple steps to ensure the purity and chromatic efficiency of the dye. Final purification ensures the product is safe for cosmetic use, removing impurities and by-products.
Applications
- Cosmetics: Basic Blue 99 is primarily used in hair dyes, both permanent and semi-permanent. It is particularly valued for its stability and ability to impart blue and black-blue shades to various hair types. It is often combined with other dyes to create custom colors. The dye is used in products such as color-depositing shampoos and pigmented conditioners.
INCI Functions:
Hair dyeing. It is an ingredient that adds a colouring to the hair that can be temporary, semi-permanent or permanent depending on what other ingredients are added to achieve the result. The pH for hair dyeing is generally between 9 and 10.
Safety
Mixed opinions on this azo dye.
The problem associated with azo dyes (monoazo or diazo) is photocatalytic degradation leading to oxidation and subsequent formation of impurities such as aromatic amines, some of which have carcinogenic activity (1).
Based on the data provided, the SCCS (Scientific Committee on Consumer Safety) is of the opinion that the use of Basic Red 51 with a maximum body concentration of 1% in non-oxidative hair dye formulations does not present a risk to the health of the consumer. For a final assessment of the use of Basic Red 51 with a maximum head concentration of 0.5% in oxidative hair dye formulations, stability data in an oxidative environment should be provided. (2)
This study confirms the danger of azo dyes with reference to Basic Red 51 (3) but when they enter the body through ingestion (3).
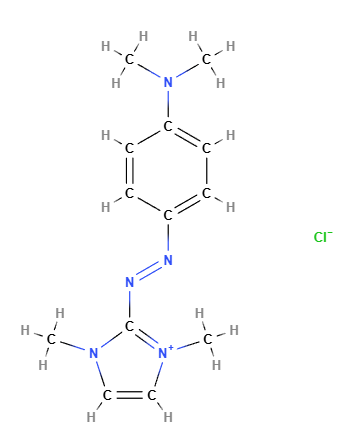 | 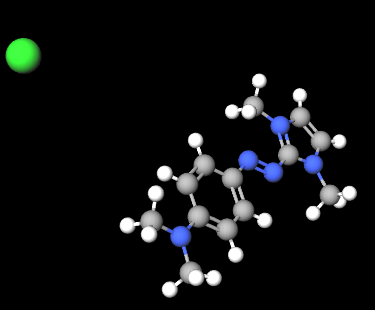 |
Molecular Formula C13H18N5.Cl
Molecular Weight 279.77 g/mol
CAS 77061-58-6
UNII A7A946JJ6I
EC Number 278-601-4
DTXSID80874049
References__________________________________________________________________________
(1) Chung KT, Stevens SE Jr, Cerniglia CE. The reduction of azo dyes by the intestinal microflora. Crit Rev Microbiol. 1992;18(3):175-90. doi: 10.3109/10408419209114557.
Abstract. Azo dyes are widely used in the textile, printing, paper manufacturing, pharmaceutical, and food industries and also in research laboratories. When these compounds either inadvertently or by design enter the body through ingestion, they are metabolized to aromatic amines by intestinal microorganisms. Reductive enzymes in the liver can also catalyze the reductive cleavage of the azo linkage to produce aromatic amines. However, evidence indicates that the intestinal microbial azoreductase may be more important than the liver enzymes in azo reduction. In this article, we examine the significance of the capacity of intestinal bacteria to reduce azo dyes and the conditions of azo reduction. Many azo dyes, such as Acid Yellow, Amaranth, Azodisalicylate, Chicago Sky Blue, Congo Red, Direct Black 38, Direct Blue 6, Direct Blue 15, Direct Brown 95, Fast Yellow, Lithol Red, Methyl Orange, Methyl Red, Methyl Yellow, Naphthalene Fast Orange 2G, Neoprontosil, New Coccine, Orange II, Phenylazo-2-naphthol, Ponceau 3R, Ponceau SX, Red 2G, Red 10B, Salicylazosulphapyridine, Sunset Yellow, Tartrazine, and Trypan Blue, are included in this article. A wide variety of anaerobic bacteria isolated from caecal or fecal contents from experimental animals and humans have the ability to cleave the azo linkage(s) to produce aromatic amines. Azoreductase(s) catalyze these reactions and have been found to be oxygen sensitive and to require flavins for optimal activity. The azoreductase activity in a variety of intestinal preparations was affected by various dietary factors such as cellulose, proteins, fibers, antibiotics, or supplementation with live cultures of lactobacilli.
(2) Scientific Committee on Consumer Safety SCCS. OPINION ON Basic Red 51 COLIPA n° B116 - 22 March 2011
(3) Chung KT, Stevens SE Jr, Cerniglia CE. The reduction of azo dyes by the intestinal microflora. Crit Rev Microbiol. 1992;18(3):175-90. doi: 10.3109/10408419209114557.
Abstract. Azo dyes are widely used in the textile, printing, paper manufacturing, pharmaceutical, and food industries and also in research laboratories. When these compounds either inadvertently or by design enter the body through ingestion, they are metabolized to aromatic amines by intestinal microorganisms. Reductive enzymes in the liver can also catalyze the reductive cleavage of the azo linkage to produce aromatic amines. However, evidence indicates that the intestinal microbial azoreductase may be more important than the liver enzymes in azo reduction. In this article, we examine the significance of the capacity of intestinal bacteria to reduce azo dyes and the conditions of azo reduction. Many azo dyes, such as Acid Yellow, Amaranth, Azodisalicylate, Chicago Sky Blue, Congo Red, Direct Black 38, Direct Blue 6, Direct Blue 15, Direct Brown 95, Fast Yellow, Lithol Red, Methyl Orange, Methyl Red, Methyl Yellow, Naphthalene Fast Orange 2G, Neoprontosil, New Coccine, Orange II, Phenylazo-2-naphthol, Ponceau 3R, Ponceau SX, Red 2G, Red 10B, Salicylazosulphapyridine, Sunset Yellow, Tartrazine, and Trypan Blue, are included in this article. A wide variety of anaerobic bacteria isolated from caecal or fecal contents from experimental animals and humans have the ability to cleave the azo linkage(s) to produce aromatic amines. Azoreductase(s) catalyze these reactions and have been found to be oxygen sensitive and to require flavins for optimal activity. The azoreductase activity in a variety of intestinal preparations was affected by various dietary factors such as cellulose, proteins, fibers, antibiotics, or supplementation with live cultures of lactobacilli.
| Evaluate |

