![]() Vitamin B2
Vitamin B2
Rating : 9.4
| Evaluation | N. Experts | Evaluation | N. Experts |
|---|---|---|---|
| 1 | 6 | ||
| 2 | 7 | ||
| 3 | 8 | ||
| 4 | 9 | ||
| 5 | 10 |
Pros:
Cognitive decline (1) Possible anti-cancer (1) Anti-arthritic (1) Cataract (1) Skin protective (1)8 pts from FRanier
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.Evaluate | Where is this found? |
| "Vitamin B2 studies" about Vitamin B2 Review Consensus 10 by FRanier (9971 pt) | 2023-Jan-21 19:55 |
| Read the full Tiiip | (Send your comment) |
Compendium of the most significant studies with reference to properties, intake, effects.
Suwannasom N, Kao I, Pruß A, Georgieva R, Bäumler H. Riboflavin: The Health Benefits of a Forgotten Natural Vitamin. Int J Mol Sci. 2020 Jan 31;21(3):950. doi: 10.3390/ijms21030950.
Abstract. Riboflavin (RF) is a water-soluble member of the B-vitamin family. Sufficient dietary and supplemental RF intake appears to have a protective effect on various medical conditions such as sepsis, ischemia etc., while it also contributes to the reduction in the risk of some forms of cancer in humans. These biological effects of RF have been widely studied for their anti-oxidant, anti-aging, anti-inflammatory, anti-nociceptive and anti-cancer properties. Moreover, the combination of RF and other compounds or drugs can have a wide variety of effects and protective properties, and diminish the toxic effect of drugs in several treatments. Research has been done in order to review the latest findings about the link between RF and different clinical aberrations. Since further studies have been published in this field, it is appropriate to consider a re-evaluation of the importance of RF in terms of its beneficial properties.
de Souza AC, Kodach L, Gadelha FR, Bos CL, Cavagis AD, Aoyama H, Peppelenbosch MP, Ferreira CV. A promising action of riboflavin as a mediator of leukaemia cell death. Apoptosis. 2006 Oct;11(10):1761-71. doi: 10.1007/s10495-006-9549-2.
Abstract. Besides having a pivotal biological function as a component of coenzymes, riboflavin appears a promissing antitumoral agent, but the underlying molecular mechanism remains unclear. In this work, we demonstrate that irradiated riboflavin, when applied at microM concentrations, induces an orderly sequence of signaling events finally leading to leukemia cell death. The molecular mechanism involved is dependent on the activation of caspase 8 caused by overexpression of Fas and FasL and also on mitochondrial amplification mechanisms, involving the stimulation of ceramide production by sphingomyelinase and ceramide synthase. The activation of this cascade led to an inhibition of mitogen activated protein kinases: JNK, MEK and ERK and survival mediators (PKB and IAP1), upregulation of the proapoptotic Bcl2 member Bax and downregulation of cell cycle progression regulators. Importantly, induction of apoptosis by irradiated riboflavin was leukaemia cell specific, as normal human lymphocytes did not respond to the compound with cell death. Our data indicate that riboflavin selectively activates Fas cascade and also constitutes a death receptor-engaged drug without harmful side effects in normal cells, bolstering the case for using this compound as a novel avenue for combating cancerous disease.
Martínez-Limón A, Calloni G, Ernst R, Vabulas RM. Flavin dependency undermines proteome stability, lipid metabolism and cellular proliferation during vitamin B2 deficiency. Cell Death Dis. 2020 Sep 7;11(9):725. doi: 10.1038/s41419-020-02929-5.
Abstract. Tumor cells adapt their metabolism to meet the energetic and anabolic requirements of high proliferation and invasiveness. The metabolic addiction has motivated the development of therapies directed at individual biochemical nodes. However, currently there are few possibilities to target multiple enzymes in tumors simultaneously. Flavin-containing enzymes, ca. 100 proteins in humans, execute key biotransformations in mammalian cells. To expose metabolic addiction, we inactivated a substantial fraction of the flavoproteome in melanoma cells by restricting the supply of the FMN and FAD precursor riboflavin, the vitamin B2. Vitamin B2 deficiency affected stability of many polypeptides and thus resembled the chaperone HSP90 inhibition, the paradigmatic multiple-target approach. In support of this analogy, flavin-depleted proteins increasingly associated with a number of proteostasis network components, as identified by the mass spectrometry analysis of the FAD-free NQO1 aggregates. Proteome-wide analysis of the riboflavin-starved cells revealed a profound inactivation of the mevalonate pathway of cholesterol synthesis, which underlines the manifold cellular vulnerability created by the flavoproteome inactivation. Cell cycle-arrested tumor cells became highly sensitive to alkylating chemotherapy. Our data suggest that the flavoproteome is well suited to design synthetic lethality protocols combining proteostasis manipulation and metabolic reprogramming.
Powers HJ, Corfe BM, Nakano E. Riboflavin in development and cell fate. Subcell Biochem. 2012;56:229-45. doi: 10.1007/978-94-007-2199-9_12.
Abstract. Riboflavin (7,8-dimethyl-10-ribitylisoalloxazine; vitamin B2) is a water-soluble vitamin, cofactor derivatives of which (FAD, FMN) act as electron acceptors in the oxidative metabolism of carbohydrate, amino acids and fatty acids and which in the reduced state can donate electrons to complex II of the electron transport chain. This means that riboflavin is essential for energy generation in the aerobic cell, through oxidative phosphorylation. The classic effects of riboflavin deficiency on growth and development have generally been explained in terms of these functions. However, research also suggests that riboflavin may have specific functions associated with cell fate determination, which would have implications for growth and development. In particular, riboflavin depletion interferes with the normal progression of the cell cycle, probably through effects on the expression of regulatory genes, exerted at both the transcriptional and proteomic level.
Henriques BJ, Lucas TG, Gomes CM. Therapeutic Approaches Using Riboflavin in Mitochondrial Energy Metabolism Disorders. Curr Drug Targets. 2016;17(13):1527-34. doi: 10.2174/1389450117666160813180812.
Abstract. Riboflavin, or vitamin B2, plays an important role in the cell as biological precursor of FAD and FMN, two important flavin cofactors which are essential for the structure and function of flavoproteins. Riboflavin has been used in therapeutic approaches of various inborn errors of metabolism, notably in metabolic disorders resulting either from defects in proteins involved in riboflavin metabolism and transport or from defects in flavoenzymes. The scope of this review is to provide an updated perspective of clinical cases in which riboflavin was used as a potential therapeutic agent in disorders affecting mitochondrial energy metabolism. In particular, we discuss available mechanistic insights on the role of riboflavin as a pharmacological chaperone for the recovery of misfolded metabolic flavoenzymes.
Bacher A, Eberhardt S, Fischer M, Kis K, Richter G. Biosynthesis of vitamin b2 (riboflavin). Annu Rev Nutr. 2000;20:153-67. doi: 10.1146/annurev.nutr.20.1.153.
Abstract. The biosynthesis of one riboflavin molecule requires one molecule of GTP and two molecules of ribulose 5-phosphate as substrates. The imidazole ring of GTP is hydrolytically opened, yielding a 4, 5-diaminopyrimidine which is converted to 5-amino-6-ribitylamino-2, 4(1H,3H)-pyrimidinedione by a sequence of deamination, side chain reduction and dephosphorylation. Condensation of 5-amino-6-ribitylamino-2,4(1H,3H)-pyrimidinedione with 3, 4-dihydroxy-2-butanone 4-phosphate obtained from ribulose 5-phosphate affords 6,7-dimethyl-8-ribityllumazine. Dismutation of the lumazine derivative yields riboflavin and 5-amino-6-ribitylamino-2,4(1H,3H)-pyrimidinedione, which is recycled in the biosynthetic pathway. The structure of the biosynthetic enzyme, 6,7-dimethyl-8-ribityllumazine synthase, has been studied in considerable detail.
Kasai S, Nakano H, Kinoshita T, Miyake Y, Maeda K, Matsui K. Intestinal absorption of riboflavin, studied by an in situ circulation system using radioactive analogues. J Nutr Sci Vitaminol (Tokyo). 1988 Jun;34(3):265-80. doi: 10.3177/jnsv.34.265.
Abstract. The intestinal absorption of riboflavin was studied using radioactive riboflavin and its analogues 8-demethylriboflavin, 3-methylriboflavin, 5'-deoxyriboflavin, 2'-deoxyriboflavin, 7,8-dimethyl-10-hydroxyethylisoalloxazine, lumiflavin, lumichrome, and riboflavin-5'-monosulfate, which were synthesized with high specific radioactivity. A specific absorption of riboflavin at dietary concentrations was confirmed using an in situ circulation system. The relation between the chemical structure of flavins and the absorption mechanism was studied using this system. The 8-demethylriboflavin, an analogue modified at benzene moiety of the isoalloxazine ring, was absorbed in a similar way to riboflavin, by dual kinetics: by a process specific for riboflavin at dietary concentrations and by simple diffusion (nonspecific absorption) predominating at higher concentrations (over 100 microM). However, 3-methylriboflavin and analogues modified at the ribityl group, including 5'-deoxyriboflavin, were absorbed only via simple diffusion even at dietary concentrations. Many flavins examined, except for 3-isobutylriboflavin, 3-carboxymethylriboflavin, lumichrome, and riboflavin-5'-monosulfate, interfered with the specific absorption of riboflavin. It was concluded from these results that one of the specific absorption processes for riboflavin is a phosphorylation-dephosphorylation process. Four water-soluble vitamins did not interfere with the specific absorption of riboflavin, indicating that these vitamins do not share a common specific absorption pathway with riboflavin.
Larrea L, Calabuig M, Roldán V, Rivera J, Tsai HM, Vicente V, Roig R. The influence of riboflavin photochemistry on plasma coagulation factors. Transfus Apher Sci. 2009 Dec;41(3):199-204. doi: 10.1016/j.transci.2009.09.006.
Abstract. Studies with riboflavin in the 1960s showed that it could be effective at inactivating pathogens when exposed to light. The principal mode of action is through electron transfer reactions, most importantly in nucleic acids. This suggested that it could act as a photosensitizer useful in the inactivation of pathogens found in blood products. Objective: To study the influence of photo-inactivation with riboflavin on the coagulation factors of plasma....Conclusions: As with other pathogen reduction procedures for plasma products, treatment with riboflavin and UV light resulted in reduction in the activity levels of several pro-coagulant factors. Coagulation inhibitors are well preserved.
Namazi N, Heshmati J, Tarighat-Esfanjani A. Supplementation with Riboflavin (Vitamin B2) for Migraine Prophylaxis in Adults and Children: A Review. Int J Vitam Nutr Res. 2015;85(1-2):79-87. doi: 10.1024/0300-9831/a000225.
Abstract. Background and aim: Migraine is a unilateral and pulsating headache associated with nausea, photophobia, vomiting, and sensitivity to light. Low vitamin B2 can lead to mitochondrial dysfunction and may have an effect on migraine pathogenesis. The aim of the present study was to carry out a review of existing evidence regarding the effects of riboflavin (vitamin B2) supplementation on migraine prophylaxis in adults and children....Conclusions: It seems that riboflavin is a safe and well-tolerated option for preventing migraine symptoms in adults, however, there is insufficient evidence to make recommendations regarding vitamin B2 as an adjunct therapy in adults and children with migraine.
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.EvaluateClose | (0 comments) |
| "Descrizione" about Vitamin B2 Review Consensus 8 by FRanier (9971 pt) | 2024-Jun-03 16:01 |
| Read the full Tiiip | (Send your comment) |
Vitamin B2 also known as Riboflavin was discovered in 1879 in milk in the form of a yellow pigment. It is an essential organic compound that plays a crucial role in numerous biological processes. It is a water-soluble vitamin and part of the B-vitamin complex, which is essential for energy production and general cellular function.
Chemical Composition and Structure
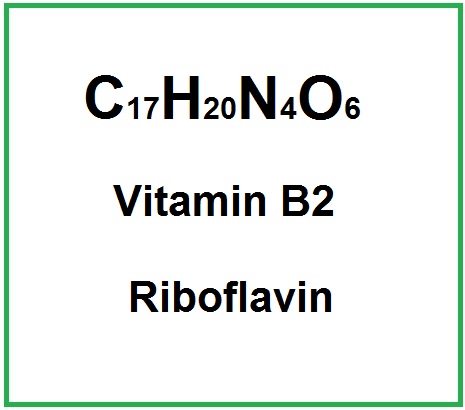
Riboflavin has the chemical formula C17H20N4O6. It is composed of a ribitol side chain and a flavin ring system. The compound's structure includes a complex aromatic ring and several hydroxyl groups, contributing to its stability and reactivity.
Physical Properties
Riboflavin typically appears as a yellow to orange-yellow crystalline powder. It is slightly soluble in water and ethanol but more soluble in dilute alkaline solutions. Riboflavin exhibits strong fluorescence, especially under ultraviolet light, which is often used as a characteristic identification property.
Biological Importance
- energy production with carbohydrate conversion.
- processing of amino acids and fats.
- activation of folic acid and vitamin B6 functions.
- controls proper functioning of the intestines, skin and mucous membranes.
Energy Production: Riboflavin is a precursor of the coenzymes flavin mononucleotide (FMN) and flavin adenine dinucleotide (FAD). These coenzymes are crucial for the oxidative phosphorylation process, where ATP is produced in mitochondria, supplying energy to cells.
Antioxidant Function: Riboflavin plays a significant role in maintaining the body's antioxidant defense system. It helps in the metabolism of glutathione, an important antioxidant that protects cells from oxidative stress.
Metabolism of Other Nutrients: Riboflavin is essential for the metabolism of carbohydrates, fats, and proteins. It assists in the conversion of these macronutrients into energy, supporting overall metabolic functions.
Cellular Growth and Function: Riboflavin is vital for normal cell growth, development, and function. It supports skin health, maintains mucous membranes, and is essential for the health of the eyes, nerves, and liver.
Dietary Sources and Supplementation
Riboflavin is found in various foods, including dairy products, eggs, lean meats, green leafy vegetables, nuts, and legumes. Due to its water-soluble nature, it is not stored in significant amounts in the body and must be consumed regularly through diet or supplements.
Chemical Industrial Synthesis Process
Preparation of reagents. The main raw materials include thioaniline and concentrated sulfuric acid.
Synthesis of thioindigoid intermediate. The production of CI 73385 begins with the reaction of thioaniline with concentrated sulfuric acid to form a thioindigoid intermediate.
Condensation. The thioindigoid intermediate is condensed with additional aromatic derivatives under controlled conditions to form the basic structure of the thioindigoid dye.
Oxidation. The condensed structure is oxidized using an oxidizing agent, such as hydrogen peroxide, to form the thioindigoid chromophore, which is responsible for the coloring properties of CI 73385.
Purification. The crude CI 73385 product is purified using techniques such as crystallization, filtration, and chromatography to remove impurities and achieve a high-purity colorant.
Stabilization. The purified CI 73385 is stabilized to ensure its stability during transportation and storage, preventing degradation and oxidation.
Quality control. The CI 73385 undergoes rigorous quality testing to ensure it meets standards for purity, color intensity, and safety. These tests include chemical analysis, spectroscopy, and microbiological testing.
Industrially it appears in the form of a water-soluble, thermostable, brown-colored powder.

What it is used for and where
Medical
It is widely used in medicine for the treatment of:
- Migraine (1)
- Cataracts
- Cornea (2)
- Rheumatoid arthritis
- Some skin diseases
- Lack of Complex II , a rare disease (3)
- Cancer prevention (4)
- Parkinson's disease (5)
Food
In the food industry it is labeled with the number E101 in the list of European food additives, a food additive whose function is to color foods deep yellow.
Cosmetics
Restricted cosmetic ingredient as IV/145 a Relevant Item in the Annexes of the European Cosmetics Regulation 1223/2009. Substance or ingredient reported:
- Riboflavin. Wording of conditions of use and warnings: Purity criteria as set out in Commission Directive 95/45/EC (E101)
Cosmetics - INCI Functions
- Colorant. This ingredient has the function of colouring the solution in which it is inserted in a temporary, semi-permanent or permanent manner, either alone or in the presence of the complementary components added for colouring.
- Skin conditioning agent - Miscellaneous. This ingredient has the task of modifying and improving the condition of the skin when it is damaged or dry, reducing flaking and restoring its elasticity.
Riboflavin is sometimes included in cosmetic formulations for its beneficial effects on skin health. It helps maintain healthy skin and can be found in various skin care products.
The most relevant studies on this vitamin have been selected with a summary of the contents:
Safety
Does not cause toxicity. Riboflavin is generally considered safe and is not associated with toxicity at typical dietary intake levels. Excess riboflavin is excreted in the urine, giving it a bright yellow color, which is a harmless side effect. As an environmentally friendly compound, riboflavin poses no significant risk to ecosystems or human health when used appropriately.
This is the opinion of EFSA :
Il gruppo EFSA ANS fornisce un parere scientifico che riesamina la sicurezza della riboflavina (E 101 (i)) e della riboflavina-5'-fosfato sodico (E 101 (ii)) che sono autorizzati come additivi alimentari nell'UE e sono stati precedentemente valutati da JECFA e dall'SCF. JECFA ha assegnato un' ADI per riboflavina e riboflavina-5'-fosfato sodico di 0-0,5 mg/kg bw/die. L' SCF ha ritenuto che l'uso di riboflavina-5'-fosfato sodico come colore alimentare non dovrebbe alterare in modo significativo l'assunzione media giornaliera di riboflavina per la quale non è stata stabilita alcuna ADI. Il gruppo di esperti scientifici non è stato fornito di un dossier presentato di recente e ha basato la sua valutazione sulle valutazioni precedenti, sulla letteratura aggiuntiva che è divenuta disponibile da allora e dai dati disponibili in seguito a una chiamata pubblica per i dati. Il gruppo di esperti scientifici ha ritenuto che la riboflavina-5'-fosfato sodico sia rapidamente defosforilata alla riboflavina libera nella mucosa intestinale e quindi metabolizzata mediante normali vie metaboliche. Il gruppo di esperti scientifici ha osservato che non sono stati osservati effetti negativi in due studi di 90 giorni nel ratto e che la riboflavina e la riboflavina-5'-fosfato non sollevano preoccupazioni rispetto alla genotossicità. Il gruppo di esperti scientifici ha altresì notato che esistono dati limitati da studi clinici in cui non sono stati riportati effetti negativi significativi. Il gruppo di esperti scientifici ha ritenuto che l'uso di riboflavina come additivi alimentari comporterà un'esposizione superiore a quella della dieta regolare e che il database disponibile non sia sufficiente per valutare se gli alti potenziali potenziali di tutte le fonti combinate abbiano effetti negativi o meno. A causa dell'assenza di studi di tossicità cancerogena/cronica e della mancanza di rilevanti studi di tossicità riproduttiva e di sviluppo, il gruppo di esperti scientifici ha ritenuto che non sia opportuno assegnare un' ADI. Il gruppo di esperti scientifici ha concluso, malgrado le incertezze della base di dati, che la riboflavina (E 101 (i)) e la riboflavina-5'-fosfato sodico (E 101 (ii)) non sono di preoccupazione per la sicurezza degli usi e dei livelli d'uso attualmente autorizzati come additivi alimentari (6).
 |  |
Molecular Formula C17H20N4O6
Molecular Weight 376.369 g/mol
CAS 83-88-5 13123-37-0
EC number: 201-507-1
UNII TLM2976OFR
DTXSID8021777
Synonyms:
- Lactoflavin
- Riboflavine
- Riboflavin
- Vitamin B2
- Food Yellow 15
- C.I. Food Yellow 15
- 7,8-Dimethyl-10-(D-ribo-2,3,4,5-tetrahydroxypentyl)isoalloxazine
- D-Ribitol, 1-deoxy-1-(3,4-dihydro-7,8-dimethyl-2,4-dioxobenzo(g)pteridin-10(2H)-yl)-
- Isoalloxazine, 7,8-dimethyl-10-(D-ribo-2,3,4,5-tetrahydroxypentyl)-
- 1-Deoxy-1-(3,4-dihydro-7,8-dimethyl-2,4-dioxobenzo[g]pteridin-10(2H)-yl)-D-ribitol
- Ovoflavin
References___________________________________________________________________
(1) Sun-Edelstein C, Mauskop A. Foods and supplements in the management of migraine headaches. Clin J Pain. 2009 Jun;25(5):446-52. doi: 10.1097/AJP.0b013e31819a6f65. Review.
Abstract. Objective: Although a wide range of acute and preventative medications are now available for the treatment of migraine headaches, many patients will not have a significant improvement in the frequency and severity of their headaches unless lifestyle modifications are made. Also, given the myriad side effects of traditional prescription medications, there is an increasing demand for "natural" treatment like vitamins and supplements for common ailments such as headaches. Here, we discuss the role of food triggers in the management of migraines, and review the evidence for supplements in migraine treatment....Conclusions: The identification of food triggers, with the help of food diaries, is an inexpensive way to reduce migraine headaches. We also recommend the use of the following supplements in the preventative treatment of migraines, in decreasing order of preference: magnesium, Petasites hybridus, feverfew, coenzyme Q10, riboflavin, and alpha lipoic acid.
(2) Meek KM, Hayes S. Corneal cross-linking - a review. Ophthalmic Physiol Opt. 2013 Mar;33(2):78-93. doi: 10.1111/opo.12032.
(3) Jain-Ghai S, Cameron JM, Al Maawali A, Blaser S, Mackay N, Robinson B, Raiman J. Complex II deficiency-A case report and review of the literature. Am J Med Genet A. 2013 Feb;161(2):285-94. doi: 10.1002/ajmg.a.35714.
(4) Wojcieszyńska D, Hupert-Kocurek K, Guzik U. Flavin-dependent enzymes in cancer prevention. Int J Mol Sci. 2012 Dec 7;13(12):16751-68. doi: 10.3390/ijms131216751.
Abstract. Statistical studies have demonstrated that various agents may reduce the risk of cancer's development. One of them is activity of flavin-dependent enzymes such as flavin-containing monooxygenase (FMO)(GS-OX1), FAD-dependent 5,10-methylenetetrahydrofolate reductase and flavin-dependent monoamine oxidase. In the last decade, many papers concerning their structure, reaction mechanism and role in the cancer prevention were published. In our work, we provide a more in-depth analysis of flavin-dependent enzymes and their contribution to the cancer prevention. We present the actual knowledge about the glucosinolate synthesized by flavin-containing monooxygenase (FMO)(GS-OX1) and its role in cancer prevention, discuss the influence of mutations in FAD-dependent 5,10-methylenetetrahydrofolate reductase on the cancer risk, and describe FAD as an important cofactor for the demethylation of histons. We also present our views on the role of riboflavin supplements in the prevention against cancer.
(5) Coimbra CG, Junqueira VB. High doses of riboflavin and the elimination of dietary red meat promote the recovery of some motor functions in Parkinson's disease patients. Braz J Med Biol Res. 2003 Oct;36(10):1409-17. doi: 10.1590/s0100-879x2003001000019.
Abstract. Abnormal riboflavin status in the absence of a dietary deficiency was detected in 31 consecutive outpatients with Parkinson's disease (PD), while the classical determinants of homocysteine levels (B6, folic acid, and B12) were usually within normal limits. In contrast, only 3 of 10 consecutive outpatients with dementia without previous stroke had abnormal riboflavin status. The data for 12 patients who did not complete 6 months of therapy or did not comply with the proposed treatment paradigm were excluded from analysis. Nineteen PD patients (8 males and 11 females, mean age +/- SD = 66.2+/-8.6 years; 3, 3, 2, 5, and 6 patients in Hoehn and Yahr stages I to V) received riboflavin orally (30 mg every 8 h) plus their usual symptomatic medications and all red meat was eliminated from their diet. After 1 month the riboflavin status of the patients was normalized from 106.4+/-34.9 to 179.2+/-23 ng/ml (N = 9). Motor capacity was measured by a modification of the scoring system of Hoehn and Yahr, which reports motor capacity as percent. All 19 patients who completed 6 months of treatment showed improved motor capacity during the first three months and most reached a plateau while 5/19 continued to improve in the 3- to 6-month interval. Their average motor capacity increased from 44 to 71% after 6 months, increasing significantly every month compared with their own pretreatment status (P < 0.001, Wilcoxon signed rank test). Discontinuation of riboflavin for several days did not impair motor capacity and yellowish urine was the only side effect observed. The data show that the proposed treatment improves the clinical condition of PD patients. Riboflavin-sensitive mechanisms involved in PD may include glutathione depletion, cumulative mitochondrial DNA mutations, disturbed mitochondrial protein complexes, and abnormal iron metabolism. More studies are required to identify the mechanisms involved.
(6) EFSA DOI: 10.2903 / j.efsa.2013.3357
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.EvaluateClose | (0 comments) |
Read other Tiiips about this object in __Italiano (2)
Component type: Chemical Main substances:
Last update: 2024-06-03 15:28:08 | Chemical Risk: |