![]() Sodium Lauryl Sulfate
Sodium Lauryl Sulfate
Rating : 4.8
| Evaluation | N. Experts | Evaluation | N. Experts |
|---|---|---|---|
| 1 | 6 | ||
| 2 | 7 | ||
| 3 | 8 | ||
| 4 | 9 | ||
| 5 | 10 |
Cons:
Allergen (1)8 pts from Ark90
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.Evaluate | Where is this found? |
| "Sodium Lauryl Sulfate studies" about Sodium Lauryl Sulfate Review Consensus 8 by Ark90 (12432 pt) | 2021-Dec-02 12:07 |
| Read the full Tiiip | (Send your comment) |
Sodium lauryl sulfate (SLS), also known as sodium lauryl sulfate or sodium dodecyl sulfate, is an anionic surfactant commonly used as a cleaning emulsifying agent in household cleaning products (laundry detergents, spray cleaners, and dishwasher detergents). The concentration of SLS in consumer products varies by product and manufacturer, but typically ranges from 0.01% to 50% in cosmetic products and 1% to 30% in cleaning products. As with most chemicals, SLS can be irritating to the eyes as a raw material or at high concentrations.Cleaning products that contain SLS have the potential to be dermal irritants if not formulated properly, but products that contain SLS are not necessarily skin irritants. The acute oral toxicity of SLS is undisputed, but it is relevant to the overall review of SLS safety. It is important to remember that the toxicity of a formulated consumer product is dictated by the formulation as a whole, not the toxicity of a single ingredient. By far the most egregious claim is that SLS is carcinogenic. The origin of this claim is uncertain, but it is likely derived from multiple misinterpretations of the scientific literature. There is no scientific evidence to support that SLS is a carcinogen. The perception that SLS is carcinogenic is often based on studies that use the ingredient to evaluate the carcinogenicity of other agents. Toxicological data support that SLS is safe for use in cleaning products when formulated to minimize the potential for irritation (1).
Sodium laurisulfate, an anionic surfactant, is known to induce roughness in the skin, and the mechanism by which it performs this action may be by disrupting wetting function (2).
This cleansing compound (SLS), a common ingredient in soaps, shampoos, toothpastes, and other skin care products, is also a commonly used test substance for the induction of skin damage. The time and quality, of skin repair are often used as indicators of various treatments, for example how, emollient ones, affect this process (3).
In a comparison of toothpastes with SLS and without SLS, those with SLS showed no significant improvement on moderate gingival inflammation (4).
References__________________________________________________________________
(1) Bondi CA, Marks JL, Wroblewski LB, Raatikainen HS, Lenox SR, Gebhardt KE. Human and Environmental Toxicity of Sodium Lauryl Sulfate (SLS): Evidence for Safe Use in Household Cleaning Products. Environ Health Insights. 2015 Nov 17;9:27-32. doi: 10.4137/EHI.S31765.
(2) Mizutani T, Mori R, Hirayama M, Sagawa Y, Shimizu K, Okano Y, Masaki H. Sodium Lauryl Sulfate Stimulates the Generation of Reactive Oxygen Species through Interactions with Cell Membranes. J Oleo Sci. 2016 Dec 1;65(12):993-1001. doi: 10.5650/jos.ess16074.
(3) Törmä H, Lindberg M, Berne B. Skin barrier disruption by sodium lauryl sulfate-exposure alters the expressions of involucrin, transglutaminase 1, profilaggrin, and kallikreins during the repair phase in human skin in vivo. J Invest Dermatol. 2008 May;128(5):1212-9. doi: 10.1038/sj.jid.5701170.
(4) Sälzer S, Rosema NA, Martin EC, Slot DE, Timmer CJ, Dörfer CE, van der Weijden GA. The effectiveness of dentifrices without and with sodium lauryl sulfate on plaque, gingivitis and gingival abrasion--a randomized clinical trial. Clin Oral Investig. 2016 Apr;20(3):443-50. doi: 10.1007/s00784-015-1535-z.
Compendium of the most significant studies with reference to properties, intake, effects.
Presley CL, Militello M, Barber C, Ladd R, Laughter M, Ferguson H, Dewey J, Pulsipher KJ, Rundle CW, Dunnick CA. The History of Surfactants and Review of Their Allergic and Irritant Properties. Dermatitis. 2021 Sep-Oct 01;32(5):289-297. doi: 10.1097/DER.0000000000000730.
Freitas R, Coppola F, Meucci V, Battaglia F, Soares AMVM, Pretti C, Faggio C. The influence of salinity on sodium lauryl sulfate toxicity in Mytilus galloprovincialis. Environ Toxicol Pharmacol. 2021 Oct;87:103715. doi: 10.1016/j.etap.2021.103715.
Lee CH, Maibach HI. The sodium lauryl sulfate model: an overview. Contact Dermatitis. 1995 Jul;33(1):1-7. doi: 10.1111/j.1600-0536.1995.tb00438.x.
Irizarry Rovira AR, Hilbish KG, Schroeder M, Boorman GA, Credille KM, Ballard D, Hanson JC, Niedenthal A. Effects of 0.5% and 2.0% Sodium Lauryl Sulfate in Male CD-1 Mice From a 3-Month Oral Gavage Toxicity Study. Toxicol Pathol. 2021 Jul;49(5):1100-1108. doi: 10.1177/01926233211004873.
Haineault C, Gourde P, Perron S, Désormeaux A, Piret J, Omar RF, Tremblay RR, Bergeron MG. Thermoreversible gel formulation containing sodium lauryl sulfate as a potential contraceptive device. Biol Reprod. 2003 Aug;69(2):687-94. doi: 10.1095/biolreprod.102.014043.
Leskur D, Bukić J, Petrić A, Zekan L, Rušić D, Šešelja Perišin A, Petrić I, Stipić M, Puizina-Ivić N, Modun D. Anatomical site differences of sodium lauryl sulfate-induced irritation: randomized controlled trial. Br J Dermatol. 2019 Jul;181(1):175-185. doi: 10.1111/bjd.17633.
Elsner P, Wilhelm D, Maibach HI. Sodium lauryl sulfate-induced irritant contact dermatitis in vulvar and forearm skin of premenopausal and postmenopausal women. J Am Acad Dermatol. 1990 Oct;23(4 Pt 1):648-52. doi: 10.1016/0190-9622(90)70268-m.
Chuang AH, Bordlemay J, Goodin JL, McPherson JC. Effect of Sodium Lauryl Sulfate (SLS) on Primary Human Gingival Fibroblasts in an In Vitro Wound Healing Model. Mil Med. 2019 Mar 1;184(Suppl 1):97-101. doi: 10.1093/milmed/usy332.
Agner T, Elsner P. Sodium lauryl sulfate: a never ending story? Br J Dermatol. 2020 Jul;183(1):13. doi: 10.1111/bjd.18787.
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.EvaluateClose | (0 comments) |
| "Descrizione" about Sodium Lauryl Sulfate Review Consensus 8 by Ark90 (12432 pt) | 2022-Oct-03 19:33 |
| Read the full Tiiip | (Send your comment) |
Sodium Lauryl Sulfate, also called SLS, or Sodium laurisulphate is a chemical compound that comes in the form of a white powder, water soluble, diluent, surfactant, therefore a component that facilitates the removal of dirt or grease particles, decreasing their adherence to surfaces and allowing water to separate them.
SLS must not be confused with SLES (Sodium laureth sulfate) because, although both are similar and have sulphuric acid and lauryl alcohol as their formula, in SLES, which is less aggressive than SLS but is ethoxylated (obtained from ethylene oxide), it is not uncommon to find in SLES ethylene oxide and 1,4-dioxane residues, chemical compounds that are considered carcinogenic (1).
A preliminary remark must be made about synthetic surfactants, which can be divided into (2):
- Anionic (SLS, ALS, SLES), which solubilises well with lipid monolayers but not with lipid bilayers model of liposomes or cytotoxicity tests towards cultured skin cells
- Nonionic amphoteric (CAPB), which solubilises well with both mono- and bilayers
- Biotensioactive (SAP), which penetrates monolayers at 1% dry mass without solubilisation, and probably penetrates bilayers, increasing their size (without solubilisation).

It is a surfactant chemical compound obtained from the reaction of sulphuric acid with lauryl alcohol by adding sodium carbonate.
It also has a 'foaming' action.
Labelled with the number E514 in the list of European food additives with the function of a surfactant diluent.
Applications: Cosmetics, soaps, toothpastes, medicinal tablets, household products
Sodium lauryl sulfate (SLS), also known as sodium laurilsulfate or sodium dodecyl sulfate, is an anionic surfactant commonly used as an emulsifying cleaning agent in household cleaning products (laundry detergents, spray cleaners, and dishwasher detergents). The concentration of SLS found in consumer products varies by product and manufacturer but typically ranges from 0.01% to 50% in cosmetic products and 1% to 30% in cleaning products. SLS can be synthetic or naturally derived. This chemical is synthesized by reacting lauryl alcohol from a petroleum or plant source with sulfur trioxide to produce hydrogen lauryl sulfate, which is then neutralized with sodium carbonate to produce SLS. The review of SLS toxicity profiles confirms that SLS is an acceptable surfactant for use in household cleaning product formulations from toxicological and sustainability perspectives. Years of anti-SLS campaigns have led to consumer concerns and confusion regarding the safety of SLS. Yet, the primary concern – that SLS has potential for being irritating to the eyes and skin – can be easily addressed by proper formula development and appropriate irritation testing performed by the product manufacturers. SLS is considered a sustainable material because of its 100% biobased content, biodegradability, and low potential to bioaccumulation. Toxicological data support that SLS is safe for use in cleaning products when formulated to minimize its irritancy potential. It is concluded that the use of SLS in cleaning product formulations does not introduce unnecessary risk to consumers or the environment because of the presence of the ingredient, and, if properly formulated and qualified, does not pose danger to human health and safety (3).
Sodium laurisulphate, an anionic surfactant, is known to induce roughness in the skin, and the mechanism by which it does this may be by disrupting the moisturising function (4).
This cleansing compound (SLS), a common ingredient in soaps, shampoos, toothpastes and other skin care products, is also a commonly used test substance for the induction of skin damage. The time and quality of skin repair are often used as indicators of various treatments, for example, how emollients affect this process (5).
In a comparison of toothpastes with SLS and without SLS, those with SLS caused irritation to the mucosa (6).
However, this study re-proposes the risk of Sodium Lauryl Sulfate (7).
As for infants, the previous roundtable recommendations concerning infant cleansing, bathing, and use of liquid cleansers were critically reviewed and updated and the quality of evidence was evaluated using the Grading of Recommendation Assessment, Development and Evaluation system. New recommendations were developed to provide guidance on diaper care and the use of emollients. A series of recommendations was formulated to characterize the attributes of ideal liquid cleansers, wipes, and emollients. In conclusion : formulations should be effectively preserved; products containing harsh surfactants, such as sodium lauryl sulfate, should be avoided (8).
From the industrial point of view, it is a product that has two great advantages :
- It is cheap
- It is easy to work with
Typical optimal characteristics of a commercial product Sodium Lauryl Sulfate
| Appearance | White powder |
| pH | 6-9 (10g/l, H2O, 20℃) |
| Density | 1.03 g/mL at 20 °C |
| Melting Point | 204-207 °C (lit.) |
| Flash point | >100°C |
| Petroleum ether soluble substances | ≤1.2% |
| Na2SO4 | ≤2.5% |
| NaCL | ≤2.5% |
| Alkalinity | ≤0.6 |
| Water | ≤2.0% |
| Total alcohols | ≥59 |
| Storage | 2-8°C |
| Chemical Risk |  |
 |  |
 |  |
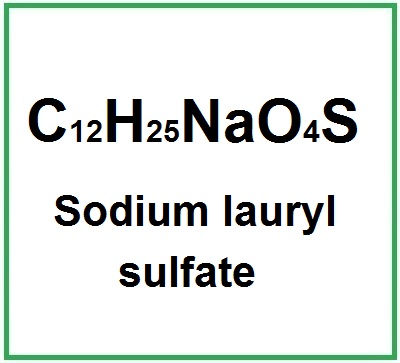
- Molecular Formula : C12H25NaO4S
- Structural formula: CH3-(CH2)11-O-SO3-Na+
- Molecular Weight : 288.38
- Exact Mass 288.137115
- CAS : 151-21-3
- UNII 368GB5141J
- EC Number: 205-788-1
- IUPAC sodium;dodecyl sulfate
- InChI=1S/C12H26O4S.Na/c1-2-3-4-5-6-7-8-9-10-11-12-16-17(13,14)15;/h2-12H2,1H3,(H,13,14,15);/q;+1/p-1
- InChl Key DBMJMQXJHONAFJ-UHFFFAOYSA-M
- SMILES CCCCCCCCCCCCOS(=O)(=O)[O-].[Na+]
- MDL number MFCD00036175
- PubChem Substance ID 24896351
- ChEBI 8984
- ICSC 0502
- RTECS WT1050000
- DSSTox Substance ID: DTXSID1026031
- FEMA number 4437
- MDL number MFCD00036175
- PubChem Substance ID
Synonyms:
- SLS
- Sodium lauryl sulphate
- Sodium dodecylsulfate
References_____________________________________________________________________
(1) Black RE, Hurley FJ, Havery DC. Occurrence of 1,4-dioxane in cosmetic raw materials and finished cosmetic products. J AOAC Int. 2001 May-Jun;84(3):666-70.
Ethylene oxide. IARC Monogr Eval Carcinog Risks Hum. 1994;60:73-159.
1,4-Dioxane. IARC Monogr Eval Carcinog Risks Hum. 1999;71 Pt 2(PT 2):589-602.
(2) Jurek I, Szuplewska A, Chudy M, Wojciechowski K. Soapwort (Saponaria officinalis L.) Extract vs. Synthetic Surfactants-Effect on Skin-Mimetic Models. Molecules. 2021 Sep 16;26(18):5628. doi: 10.3390/molecules26185628.
(3) Bondi CA, Marks JL, Wroblewski LB, Raatikainen HS, Lenox SR, Gebhardt KE. Human and Environmental Toxicity of Sodium Lauryl Sulfate (SLS): Evidence for Safe Use in Household Cleaning Products. Environ Health Insights. 2015 Nov 17;9:27-32. doi: 10.4137/EHI.S31765.
(4) Mizutani T, Mori R, Hirayama M, Sagawa Y, Shimizu K, Okano Y, Masaki H. Sodium Lauryl Sulfate Stimulates the Generation of Reactive Oxygen Species through Interactions with Cell Membranes. J Oleo Sci. 2016 Dec 1;65(12):993-1001. doi: 10.5650/jos.ess16074.
(5) Törmä H, Lindberg M, Berne B. Skin barrier disruption by sodium lauryl sulfate-exposure alters the expressions of involucrin, transglutaminase 1, profilaggrin, and kallikreins during the repair phase in human skin in vivo. J Invest Dermatol. 2008 May;128(5):1212-9. doi: 10.1038/sj.jid.5701170.
(6) Rantanen I, Jutila K, Nicander I, Tenovuo J, Söderling E. The effects of two sodium lauryl sulphate-containing toothpastes with and without betaine on human oral mucosa in vivo. Swed Dent J. 2003;27(1):31-4.
(8) Blume-Peytavi U, Lavender T, Jenerowicz D, Ryumina I, Stalder JF, Torrelo A, Cork MJ. Recommendations from a European Roundtable Meeting on Best Practice Healthy Infant Skin Care. Pediatr Dermatol. 2016 May;33(3):311-21. doi: 10.1111/pde.12819.
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.EvaluateClose | (0 comments) |
Read other Tiiips about this object in __Italiano (2)
Component type: Chemical Main substances: Last update: 2023-07-26 11:42:14 | Chemical Risk: |