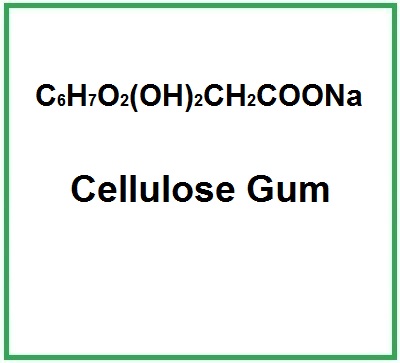
Cellulose Gum or Carboxymethylcellulose is a chemical compound obtained from cellulose.
Carboxymethylcellulose (CMC) is a derivative of cellulose, a natural polysaccharide found in the cell walls of plants. It is used in cosmetics and personal care products as a thickening, stabilizing, and film-forming agent. Due to its ability to enhance the viscosity and texture of formulations, CMC is widely used in creams, lotions, gels, and hair products. It is also appreciated for its moisturizing properties and its ability to form a protective film on the skin.
Chemical Composition and Structure
Carboxymethylcellulose is a modified cellulose where carboxymethyl groups (-CH2-COOH) are added to its polymer structure. Its chemical formula varies depending on the degree of substitution (the number of carboxymethyl groups added) and the length of the cellulose chain. CMC is water-soluble, making it ideal for use in a wide range of water-based cosmetic products.
Physical Properties
Carboxymethylcellulose appears as a white or yellowish powder, soluble in both cold and hot water, where it forms a viscous solution. Due to its ability to bind water, CMC can increase the viscosity of formulations and provide a richer, creamier texture, making it ideal for moisturizing cosmetic products.
Production Process
CMC is produced through a chemical modification process of natural cellulose. The cellulose is treated with monochloroacetic acid in the presence of alkali, introducing carboxymethyl groups along the cellulose polymer chain, enhancing its solubility and functional properties.
The name describes the structure of the molecule
- "Carboxy" refers to the carboxyl functional group (-COOH) that is introduced into the cellulose molecule. This group gives the molecule anionic or negative properties.
- "Methyl" indicates the introduction of a methyl group (-CH3) alongside the carboxyl group during derivatization. This group is attached to the carboxyl group, forming the carboxymethyl group.
- "Cellulose" is the natural sugar polymer from which CMC is derived. Cellulose is a polysaccharide made up of glucose units and is found in the cell walls of plants.
Description of raw materials used in production
- Cellulose extracted from plant sources like wood or cotton fibers.
- Monochloroacetic anhydride that provides the carboxymethyl group.
Step-by-step summary of its industrial chemical synthesis process.
- Cellulose is purified and reduced to a powdery form.
- The powdered cellulose is then treated with an alkaline solution, usually sodium hydroxide, to activate the hydroxyl groups.
- Monochloroacetic anhydride is then added to the mixture, where it reacts with the activated hydroxyl groups of cellulose to form the carboxymethyl group.
- The reaction is controlled to achieve the desired degree of substitution.
- The product is then neutralized, typically with hydrochloric acid.
- The CMC is then purified, filtered, and dried to achieve the desired final form.
It appears as an odourless and tasteless white-grey-yellow powder.

It is a chemical compound obtained from cellulose and it appears as an odourless and tasteless white-grey-yellow powder.
What it is used for and where
Food
In the food industry (1), it is a thickening and stabilising agent, and since the 1980s it has entered the oenological field (2).
Ingredient included in the list of European food additives as E466
Cosmetics
Binder agent. Ingredient that is used in cosmetic, food and pharmaceutical products as an anti-caking agent with the function of making the product in which it is incorporated silky, compact and homogenous. The binder, either natural such as mucilage, gums and starches or chemical, may be in the form of a powder or liquid.
Emulsion stabilizer. Emulsions are thermodynamically unstable. Emulsion stabilisers improve the formation and stability of single and double emulsions. It should be noted that in the structure-function relationship, molar mass plays an important role.
Film-forming agent. It produces a continuous ultra-thin film with an optimal balance of cohesion, adhesion and stickiness on the skin or hair to counteract or limit damage from external phenomena such as chemicals, UV rays and pollution.
Fragrance. It plays a decisive and important role in the formulation of cosmetic products as it provides the possibility of enhancing, masking or adding fragrance to the final product, increasing its marketability. The consumer always expects to find a pleasant or distinctive scent in a cosmetic product.
Viscosity control agent. It controls and adapts viscosity to the required level for optimal chemical and physical stability of the product and dosage in gels, suspensions, emulsions, solutions.
Other uses
In the mining sector it is used as an additive for drilling muds and is widely used in the production of paper, textiles, paints and more.
Health and Safety Considerations
Safety in Use
Carboxymethylcellulose is considered safe for use in cosmetic and personal care products. It is well tolerated by the skin and is not known to cause significant irritation or sensitization. It is also used in the food and pharmaceutical industries, further confirming its high safety profile.
Allergic Reactions
Allergic reactions to CMC are extremely rare. However, it is always recommended to perform a patch test before using products containing this ingredient, especially for individuals with very sensitive skin.
Toxicity and Carcinogenicity
There is no evidence that Carboxymethylcellulose is toxic or carcinogenic. It is widely used in both cosmetic and food applications without known health risks when used in recommended concentrations.
Environmental and Safety Considerations
CMC is biodegradable and considered environmentally safe, as it is derived from a renewable natural resource. The production process has a relatively low environmental impact, making it an eco-friendly ingredient for cosmetic formulations.
Regulatory Status
Carboxymethylcellulose is approved for use in cosmetic products in many regions, including the European Union and the United States. It is also approved for use in food and pharmaceuticals, confirming its wide range of applications and safety.
Cellulose gum studies
- Molecular Formula : C8H16O8
- Molecular Weight: 240.208 g/mol
- CAS: 9000-11-7
- EC Number:618-326-2
- FEMA Number: 2239
Synonyms:
- Carboxymethyl Cellulose
- Cellulose, Carboxymethyl
- Carboxymethylcellulose
- carmellose sodium
- Carboxymethylcellulose Sodium
- Cellolax
- Carboxymethylcellulose, Sodium
References________________________________________________________________________
(1) Regolamento (CE) N° 1333/2008 del 16 dicembre 2008 sugli additivi alimentari italiani
Determination of carboxyméthycellulose in food products - H.D Graham, Journal of food science 1971, p 1052-1055.
(2) Stabilisation tartrique des vins par la carboxyméthylcellulose - Bulletin de l’OIV 2001, vol 74, n°841-842, p151-159.
![]() Carboxymethyl cellulose
Carboxymethyl cellulose