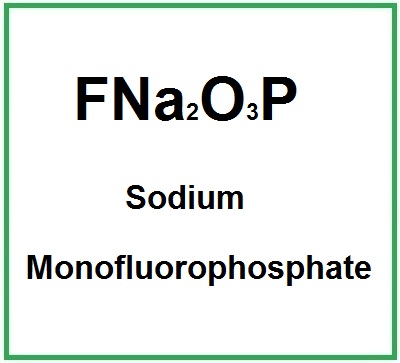
![]() Sodium Monofluorophosphate
Sodium Monofluorophosphate
Rating : 7
| Evaluation | N. Experts | Evaluation | N. Experts |
|---|---|---|---|
| 1 | 6 | ||
| 2 | 7 | ||
| 3 | 8 | ||
| 4 | 9 | ||
| 5 | 10 |
Cons:
Cosmetics Regulation provisions (1)8 pts from Ark90
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.Evaluate | Where is this found? |
| "Sodium Monofluorophosphate studies" about Sodium Monofluorophosphate by Ark90 (12432 pt) | 2022-Oct-15 19:11 |
| Read the full Tiiip | (Send your comment) |
Compendium of the most significant studies with reference to properties, intake, effects.
Ramos MCC, Rebelo MAB, Rebelo Vieira JM, Miranda LFB, Tabchoury CPM, Cury JA. Fluoride toothpaste, sanitary surveillance and the SUS: the case of Manaus-AM, Brazil. Rev Saude Publica. 2022 Mar 21;56:9. doi: 10.11606/s1518-8787.2022056003636.
Abstract. Objective: To determine the anticaries potential of toothpastes distributed by the primary health care public clinics (UBS) of Manaus, AM. Methods: Ninety-nine tubes of toothpaste from four commercial brands were collected from October 7, 2019 to October 11, 2019 in 16 UBS. They were assigned a code by brand and source UBS. According to the information on the packaging, the four brands and their batches were formulated with sodium monofluorophosphate (Na2FPO3) and most (91%) had calcium carbonate (CaCO3) as an abrasive. We determined the concentrations of total fluoride (TF = TSF + InsF) and total soluble fluoride (TSF = F ions- or FPO32-), to certify whether they were in compliance with resolution ANVISA RDC No. 530 (maximum of 1,500 ppm TF) and whether they had anticaries potential (minimum of 1,000 ppm TSF). The analyses were performed with a ion- specific electrode. Results: The concentrations (ppm F) of TF [mean; standard deviation (SD); n] found in toothpaste brands A (1,502.3; SD = 45.6; n = 33), B (1,135.5; SD = 52.7; n = 48) and D (936.8; SD = 20.5; N = 8) were close to those stated on the package, 1,500, 1,100 and 1,000 ppm F, respectively. In toothpaste C, we found a mean of 274.1 ppm (SD = 219.7; n = 10) of TF, which diverges from the declared concentration of 1,500 ppm F. In addition, the five tubes of lot no. 11681118 of toothpaste C did not contain fluoride. Regarding TSF, with the exception of toothpaste D (937.9; SD = 40.29), the others had a lower concentration than their respective TF. Conclusion: We found serious problems of quantity and quality of fluoride in toothpaste distributed by the SUS in Manaus, which shows the need for surveillance of these products and confirms the urgency of revising resolution RDC No. 530.
Kharaeva ZF, Mustafaev MS, Khazhmetov AV, Gazaev IH, Blieva LZ, Steiner L, Mayer W, Luca C, Korkina LG. Anti-Bacterial and Anti-Inflammatory Effects of Toothpaste with Swiss Medicinal Herbs towards Patients Suffering from Gingivitis and Initial Stage of Periodontitis: from Clinical Efficacy to Mechanisms. Dent J (Basel). 2020 Jan 15;8(1):10. doi: 10.3390/dj8010010.
Abstract. Objective: To distinguish clinical effects and mechanisms of sodium monofluorophosphate plus xylitol and herbal extracts of Swiss medicinal plants (Chamomilla recutita, Arnica montana, Echinacea purpurea, and Salvia officinalis). Materials and methods: A 2-month-long comparative clinical study of toothpaste containing 1450 ppm sodium monofluorophosphate and xylitol (control, 15 patients) and toothpaste additionally containing extracts of the medicinal herbs (experiment, 35 patients) was performed on patients with gingivitis and the initial stage of periodontitis. Clinical indices of gingivitis/periodontitis were quantified by Loe & Silness's, CPITN, OHI-S, and PMA indexes. The pro-inflammatory and anti-inflammatory interleukins, nitrites/nitrates, total antioxidant activity, and bacterial pattern characteristic for gingivitis and periodontitis were quantified in the gingival crevicular fluid and plaque. In the in vitro tests, direct anti-bacterial effects, inhibition of catalase induction in Staphylococcus aureus, in response to oxidative burst of phagocytes, and intracellular bacterial killing were determined for the toothpastes, individual plant extracts, and their mixture. Results: Experimental toothpaste was more efficient clinically and in the diminishing of bacterial load specific for gingivitis/periodontitis. Although the control toothpaste exerted a direct moderate anti-bacterial effect, herbal extracts provided anti-inflammatory, anti-oxidant, direct, and indirect anti-bacterial actions through inhibition of bacterial defence against phagocytes. Conclusions: Chemical and plant-derived anti-bacterials to treat gingivitis and periodontitis at the initial stage should be used in combination amid their different mechanisms of action. Plant-derived actives for oral care could substitute toxic chemicals due to multiple modes of positive effects.
Tao D, Ling MR, Feng XP, Gallob J, Souverain A, Yang W, Alavi A. Efficacy of an anhydrous stannous fluoride toothpaste for relief of dentine hypersensitivity: A randomized clinical study. J Clin Periodontol. 2020 Aug;47(8):962-969. doi: 10.1111/jcpe.13305.
Abstract. Aim: To compare efficacy of an anhydrous 0.454% w/w stannous fluoride/sodium fluoride toothpaste (Test) versus a sodium monofluorophosphate toothpaste (Negative control) and a stannous chloride/sodium fluoride toothpaste (Positive control) for dentine hypersensitivity relief after 8 weeks' twice-daily use....Conclusion: While twice-daily use of Test toothpaste significantly reduced dentine hypersensitivity from baseline, there was no significant advantage over negative or positive controls. © 2020 The Authors. Journal of Clinical Periodontology published by John Wiley & Sons Ltd.
Creeth J, Gallob J, Sufi F, Qaqish J, Gomez-Pereira P, Budhawant C, Goyal C. Randomised clinical studies investigating immediate and short-term efficacy of an occluding toothpaste in providing dentine hypersensitivity relief. BMC Oral Health. 2019 Jun 4;19(1):98. doi: 10.1186/s12903-019-0781-x.
Abstract. Background: Dentine hypersensitivity (DH) can occur after gum recession or enamel loss and may impact quality of life. Treatments include toothpastes that decrease DH by occluding dentine tubules. One effective occluding ingredient used in toothpastes is stannous fluoride (SnF2), but this can be unstable in aqueous formulation. These three studies aimed to characterise the short-term effects of an experimental, anhydrous SnF2 dentifrice on DH. Methods: Three examiner-blind, parallel-group studies evaluated DH in participants with the condition after a single brushing and after 3d brushing with an experimental anhydrous 0.454% SnF2/polyphosphate toothpaste (Test) or a toothpaste containing 0.76% sodium monofluorophosphate (Control). Test treatment participants brushed two pre-identified sensitive teeth first, then their remaining dentition for ≥1 min ('focused brushing'). Control treatment participants brushed their whole dentition for ≥1 min. DH was measured after single brushing and after 3d twice-daily use, via evaporative (air) (Schiff Sensitivity Scale) and tactile (Yeaple probe) stimuli and analysed using an ANCOVA model. Results: In all studies, after 3d treatment, the Test toothpaste/brushing regimen significantly reduced DH compared to the Control regimen by both evaporative and tactile stimuli assessment (p < 0.0001 for all). The Test regimen also significantly reduced DH from baseline at both time-points by both measures in all studies (p < 0.0001 for all). Mean Schiff sensitivity score differences (95% confidence intervals) between Test and Control regimens after 3d were: Study 1: - 0.45 (- 0.577, - 0.319); Study 2: - 0.40 (- 0.505, - 0.300); Study 3: - 1.31 (- 1.500, - 1.128). Mean tactile score differences were: Study 1: 11.30 (7.927, 14.662); Study 2: 3.57 (2.531, 4.614); Study 3: 24.54 (20.349, 28.736). After single use, in Studies 2 and 3, the Test toothpaste/brushing regimen significantly reduced DH versus Control by both measures (p < 0.001 for all); in Study 1, treatment differences were not significant. Toothpastes were generally well-tolerated. Conclusions: Taken together, these studies indicated focused brushing with an experimental anhydrous 0.454% SnF2/polyphosphate toothpaste reduces DH compared to brushing with a conventional toothpaste after single use, with greater reduction after 3d.
Cagetti MG, Cocco F, Wierichs RJ, Wolf TG, Salerno C, Arghittu A, Campus G. Efficacy of HAF toothpastes in primary and permanent dentitions. A 2-years triple-blind RCT. J Dent. 2022 Jun;121:104049. doi: 10.1016/j.jdent.2022.104049.
Abstract. Objectives: The aim of this RCT was to compare the caries preventive efficacy and the slowing down of previous caries lesions of toothpastes containing fluoride biomimetic hydroxyapatite (HA) complex compared to sodium monofluorophosphate fluoridated toothpastes in Italian schoolchildren. To validate this hypothesis a triple-blind randomized clinical trial was designed....Conclusions; The use of toothpastes containing biomimetic hydroxyapatite and fluoride reduces caries increment in children over a period of 2 years more than traditional fluoridated toothpastes. Copyright © 2022. Published by Elsevier Ltd.
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.EvaluateClose | (0 comments) |
| "Descrizione" about Sodium Monofluorophosphate Review Consensus 8 by Ark90 (12432 pt) | 2024-Oct-04 16:22 |
| Read the full Tiiip | (Send your comment) |
Sodium monofluorophosphate is an inorganic chemical compound produced synthetically by the reaction of sodium fluoride with sodium metaphosphate or the reaction of disodium phosphate or tetrasodium pyrophosphate with hydrogen fluoride.
The name defines the structure of the molecule:
- Sodium is a chemical element with the symbol Na (from the Latin "Natrium") and the atomic number 11. In the context of sodium monofluorophosphate, it indicates the presence of a sodium salt.
- Monofluorophosphate is a compound that consists of a phosphate group (PO4) and a fluorine atom (F). It is commonly used as a source of fluoride in oral care products, such as toothpaste and mouthwash. Fluoride helps prevent tooth decay by strengthening the enamel and inhibiting the growth of caries-causing bacteria.
Description of the raw materials used in its production:
- Sodium hydroxide (NaOH) - Sodium hydroxide is used as a source of sodium ions in the synthesis of sodium monofluorophosphate. It provides the necessary sodium component for the compound.
- Phosphoric acid (H3PO4) - Phosphoric acid is reacted with sodium fluoride to produce sodium monofluorophosphate. It serves as a source of phosphate ions in the compound.
- Sodium fluoride (NaF) - Sodium fluoride is combined with phosphoric acid to form sodium monofluorophosphate. It provides the fluoride component, which is essential for the compound's oral care benefits.
The synthesised extraction process takes place in four steps:
- Reaction. In the first step of the process, phosphorus pentachloride reacts with hydrogen fluoride to form phosphorus pentafluoride:
- Hydrolysis. Phosphorous pentafluoride is hydrolysed and reacted with water. Phosphoric acid and hydrogen fluoride are thus formed.
- Formation of monofluorophosphoric acid. Phosphoric acid is reacted again with hydrogen fluoride to form monofluorophosphoric acid.
- Neutralisation. In the fourth and final step, monofluorophosphoric acid is neutralised with sodium hydroxide to form sodium monofluorophosphate.
It appears in the form of a white powder, easily soluble in water at 42% at 25°C and strongly hygroscopic, Incompatible with phosphorus oxides, strong oxidising agents.

What it is used for and where
Dentistry
Used in toothpastes for enamel protection, anti-caries agent, dentine desensitisation. Sodium fluoride substitute. Sodium monofluorophosphate generally accounts for 0.7-0.76% in toothpaste ingredients and has virtually replaced sodium fluoride. Fungicide and preservative.
Safety
Maximum concentration in the ready-to-use preparation: 0.15% calculated as Fluorine. When mixed with other fluorine compounds permitted by this annex, the total concentration of F shall not exceed 0.15%.
Formulation of conditions of use and warnings: Contains sodium monofluorophosphate. For any toothpaste containing 0.1 to 0.15 percent fluoride, unless it is already labeled as contraindicated for children (e.g., "for adult use only"), the following labeling is mandatory: "Children 6 years of age or younger: Use a minimum amount for supervised brushing to minimize ingestion." In case of fluoride intake from other sources, consult a dentist or physician."
Cosmetics
It is a restricted ingredient III/27 as a Relevant Item in the Annexes of the European Cosmetics Regulation No. 1223/2009.
Anti-plaque agent. It is an ingredient that has the property of preventing the occurrence of caries by fighting the bacteria responsible for acid corrosion of teeth.
Oral hygiene agent. This ingredient can be inserted into the oral cavity of to improve and/or maintain its oral hygiene and health, to prevent or improve a disorder of the teeth, gums, mucosa.
Other uses
Metal surface cleaning, special glass production, drinking water fluorination, concrete corrosion inhibitor
Commercial applications
Fluoridating Agent in Toothpastes. Sodium Monofluorophosphate is used in toothpastes to help prevent tooth decay.
Component in Oral Care Products. Used in mouthwashes and other oral care products to promote tooth health.
Preservative Agent for Dental Materials. It can be used in dental materials as a preservative and stabilizing agent.
Ingredient in Pharmaceutical Preparations. Used in some pharmaceutical preparations for its antimicrobial properties.
For more information:
Sodium monofluorophosphate studies
Typical characteristics of the commercial product Sodium monofluorophosphate for use in toothpastes
| Appearance | White powder |
| pH | 6.5-8.0 |
| Melting Point | 625°C |
| Apparent Density | 1.5 g/cm3 |
| PSA | 73.00000 |
| LogP | 0.92500 |
| Na | ≥32.0% |
| Arsenic | ≤0.0003% |
| Lead | ≤0.0001% |
| Heavy metal | ≤0.005% |
| P (P2O5) | ≥49.0% |
| Combined fluorine | ≥12.49% |
| Free fluorine | ≤0.5% |
| Total F | ≥13.0% |
| F Combined in PO3F2- | ≥12.54% |
| Loss on dry (105℃/2H) | ≤0.2% |
| Whiteness (70-90umGranularity) | ≥94% |
| Transmission (15% Aq.solution) | ≥89% |
| Mesh granularity | 90% pass 100-200 mesh |
| Shelf life | 2 years |
| Chemical Safety |  |
 |  |
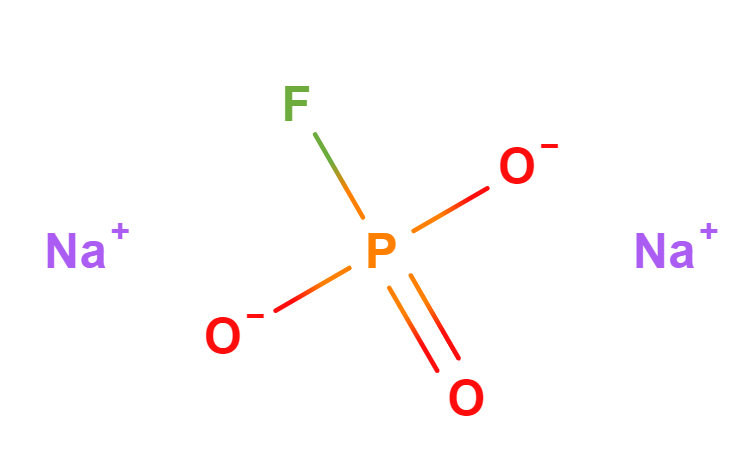 | 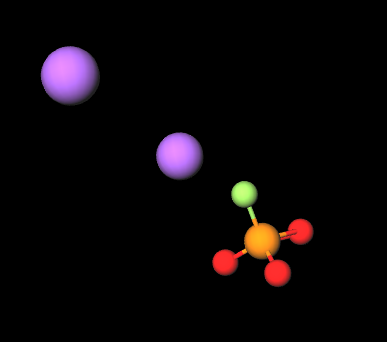 |
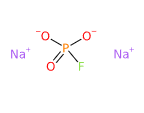
- Molecular Formula FNa2O3P Na2PFO3
- Molecular Weight 143.949 g/mol
- Exact Mass 143.93600
- CAS 10163-15-2
- UNII: C810JCZ56Q
- EC Number: 233-433-0
- DSSTox Substance ID DTXSID70872554 DTXSID40872566
- IUPAC disodium;fluoro-dioxido-oxo-λ5-phosphane
- InChl=1S/FH2O3P.2Na/c1-5(2,3)4;;/h(H2,2,3,4);;/q;2*+1/p-2
- InChl Key BFDWBSRJQZPEEB-UHFFFAOYSA-L
- SMILES [O-]P(=O)([O-])F.[Na+].[Na+]
- MDL number MFCD00014248
- PubChem Substance ID
- ChEBI 86431
- RTECS TE6130000
- NCI C61945
- RXCUI 56501
Synonyms:
- Disodium monofluorophosphate
- disodium;fluoro-dioxido-oxo-λ5-phosphane
- Sodium fluorophosphate
- Disodium fluorophosphate
- Sodium phosphorofluoridate
- Sodium monofluorophosphate [USP]
- disodium fluoro-dioxido-oxophosphorane
- Fluoridophosphoric acid disodium salt
- Phosphorofluoridic acid disodium salt
- Phosphorofluoridic acid, disodium salt
- Fluorophosphoric acid, sodium salt
- Disodium phosphorofluoridate
- dinatriumfluorophosphat
References____________________________________________________________________
Satyawan G. Damle, D Deoyani, Hiteshwar Bhattal, Renu Yadav, Ashish Lomba Comparative efficacy of dentifrice containing sodium monofluorophosphate + calcium glycerophosphate and non-fluoridated dentifrice: A randomized, double-blind, prospective study Dent Res J (Isfahan) 2012 Jan-Mar
Abstract. Background: The efficacy of fluoridated dentifrices in caries prevention has been well documented and research into various formulations continues for a more effective dentifrice. This study evaluated the anti-caries and anti-plaque efficacy of a dentifrice containing sodium monofluorophosphate (1000 ppm) and calcium glycerophosphate, and compared it with a non-fluoridated dentifrice....Conclusion: Results revealed that the test dentifrice was effective in inhibiting the progression of plaque and control of dental caries as compared to the placebo dentifrice.
Grewal N, Sharma N, Kaur N. Surface remineralization potential of nano-hydroxyapatite, sodium monofluorophosphate, and amine fluoride containing dentifrices on primary and permanent enamel surfaces: An in vitro study. J Indian Soc Pedod Prev Dent. 2018 Apr-Jun;36(2):158-166. doi: 10.4103/JISPPD.JISPPD_142_17
Abstract. Background: Organic amine fluorides and nano-hydroxyapatite dentifrices have shown remineralization potential in various studies. However, there is a lack of direct comparison between amine fluoride and nano-hydroxyapatite with conventional inorganic fluorides as sodium monofluorophosphate. Aim: The aim of the study is to evaluate remineralizing efficacy of the three dentifrices on both primary and permanent enamel surfaces.....Conclusions: Nano-hydroxyapatite exhibited highest remineralization potential in terms of mineral gain followed by amine fluoride and sodium monofluorophosphate dentifrice.
Gallob J, Sufi F, Amini P, Siddiqi M, Mason S. A randomised exploratory clinical evaluation of dentifrices used as controls in dentinal hypersensitivity studies. J Dent. 2017 Sep;64:80-87. doi: 10.1016/j.jdent.2017.06.009. Epub 2017 Jun 23.
Parkinson CR, Siddiqi M, Mason S, Lippert F, Hara AT, Zero DT. Anticaries Potential of a Sodium Monofluorophosphate Dentifrice Containing Calcium Sodium Phosphosilicate: Exploratory in situ Randomized Trial. Caries Res. 2017;51(2):170-178. doi: 10.1159/000453622. Epub 2017 Feb 21.
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.EvaluateClose | (0 comments) |
Read other Tiiips about this object in __Italiano (4)
Component type: Chemical Main substances: Last update: 2023-03-30 11:51:30 | Chemical Risk: |