![]() Limonene
Limonene
Rating : 7.3
| Evaluation | N. Experts | Evaluation | N. Experts |
|---|---|---|---|
| 1 | 6 | ||
| 2 | 7 | ||
| 3 | 8 | ||
| 4 | 9 | ||
| 5 | 10 |
Pros:
Antioxidant (1)Cons:
Specific allergy (1)10 pts from Ark90
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.Evaluate | Where is this found? |
| "Limonene studies" about Limonene Review Consensus 17 by Ark90 (12432 pt) | 2021-Dec-20 11:13 |
| Read the full Tiiip | (Send your comment) |
Compendium of the most significant studies with reference to properties, intake, effects.
Araújo-Filho HG, Dos Santos JF, Carvalho MTB, Picot L, Fruitier-Arnaudin I, Groult H, Quintans-Júnior LJ, Quintans JSS. Anticancer activity of limonene: A systematic review of target signaling pathways. Phytother Res. 2021 Sep;35(9):4957-4970. doi: 10.1002/ptr.7125.
Abstract. Limonene (LIM) is a monoterpene, which is abundant in essential oils of Citrus fruits peels (Rutaceae). More recently, LIM, as a potential natural anticancer compound, has attracted major attention and exerted a chemopreventive activity, stimulating the detoxification of carcinogenic compounds and limiting tumor growth and angiogenesis in various cancer models.
Sun C, Theodoropoulos C, Scrutton NS. Techno-economic assessment of microbial limonene production. Bioresour Technol. 2020 Mar;300:122666. doi: 10.1016/j.biortech.2019.122666.
Abstract. A techno-economic analysis is carried out for one such process producing limonene from sugar at an industrial plant scale to assess potential economic viability. A conceptual design of the process is developed, in which a gas stripping-solvent scrubbing method is chosen for recovering limonene from bioreactors based on consideration of payback time and process operability.
Garre A, Espín JF, Huertas JP, Periago PM, Palop A. Limonene nanoemulsified with soya lecithin reduces the intensity of non-isothermal treatments for inactivation of Listeria monocytogenes. Sci Rep. 2020 Feb 27;10(1):3656. doi: 10.1038/s41598-020-60571-9.
Abstract. Treatments with a high heating rate (20 °C/min) are more effective than those with a slow heating rate (1 °C/min). This investigation demonstrates that the addition of nanoemulsified D-limonene can greatly reduce the intensity of the thermal treatments currently applied in the food industry. Hence, it can improve the product quality without impacting its safety.
Santana HSR, de Carvalho FO, Silva ER, Santos NGL, Shanmugam S, Santos DN, Wisniewski JO, Junior JSC, Nunes PS, Araujo AAS, de Albuquerque Junior RLC, Dos Santos MRV. Anti-Inflammatory Activity of Limonene in the Prevention and Control of Injuries in the Respiratory System: A Systematic Review. Curr Pharm Des. 2020;26(18):2182-2191. doi: 10.2174/1381612826666200320130443.
Abstract. The purpose of this article is to provide an overview of the anti-inflammatory activity of limonene and its capacity to prevent and control respiratory system injuries.
Gentile D, Berliocchi L, Russo R, Bagetta G, Corasaniti MT. Effects of the autophagy modulators d-limonene and chloroquine on vimentin levels in SH-SY5Y cells. Biochem Biophys Res Commun. 2020 Dec 17;533(4):764-769. doi: 10.1016/j.bbrc.2020.09.073.
Abstract. Here, we report that this monoterpene rapidly enhances Ca2+ levels in SH-SY5Y cells; yet this effect does not lead to calpain- or caspase-mediated proteolysis of α-spectrin, nor calpain activity is required for the established enhancement of LC3-II levels by d-limonene.
Ciriminna R, Lomeli-Rodriguez M, Demma Carà P, Lopez-Sanchez JA, Pagliaro M. Limonene: a versatile chemical of the bioeconomy. Chem Commun (Camb). 2014 Dec 18;50(97):15288-96. doi: 10.1039/c4cc06147k.
Abstract. We anticipate that the expansion in uses for limonene will translate into increasing production and use of this relevant natural product, especially for advanced applications.
Justino de Araújo AC, Freitas PR, Rodrigues Dos Santos Barbosa C, Muniz DF, Rocha JE, Albuquerque da Silva AC, Datiane de Morais Oliveira-Tintino C, Ribeiro-Filho J, Everson da Silva L, Confortin C, Amaral WD, Deschamps C, Barbosa-Filho JM, Ramos de Lima NT, Tintino SR, Melo Coutinho HD. GC-MS-FID characterization and antibacterial activity of the Mikania cordifolia essential oil and limonene against MDR strains. Food Chem Toxicol. 2020 Feb;136:111023. doi: 10.1016/j.fct.2019.111023.
Abstract. The present study evaluated the effect of the essential oil of Mikania cordifolia (EOMc) and its major constituent limonene alone or associated with antibacterial drugs against Multidrug Resistant Bacteria (MDR). To evaluate the antibacterial activity, the minimum inhibitory concentrations (MIC) of the oil and limonene against Pseudomonas aeruginosa, Escherichia coli and Staphylococcus aureus were determined.
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.EvaluateClose | (0 comments) |
| "Descrizione" about Limonene Review Consensus 10 by Ark90 (12432 pt) | 2024-Sep-20 19:03 |
| Read the full Tiiip | (Send your comment) |
Limonene (4-isopropenyl-1-methylcyclohexene) is a monoterpene hydrocarbon, one of the most frequently found in the plant kingdom, synthesised naturally from plants through a process with geranyl pyrophosphate.
It has two optical isomers:
- d-limonene
- l-limonene, a slightly yellow transparent liquid with a pleasant lemon scent which is produced industrially by cold pressing of generally dried citrus pulp and peel or by mechanical vaporization processes such as Soxhlet.
Because of its olfactory characteristics and its wide availability in nature, it is a flavouring terpene that is included in beverages, foodstuffs, home cosmetics and personal hygiene products in order to impart a pleasant fragrance to the product.
Limonene is an organic compound found in the essential oils of citrus fruits, known for its fresh, citrus scent. It is commonly used as a fragrance in cosmetics, skin care products, and perfumes.
Chemical Composition and Structure
The chemical composition of Limonene includes:
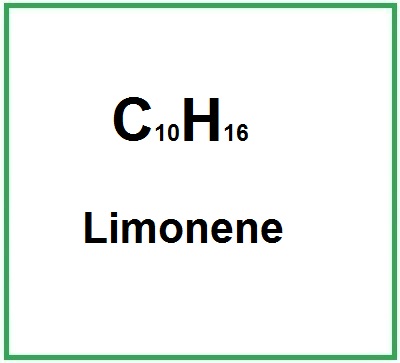
- Structure: It is a monoterpene with the chemical formula C10H16, characterized by a cyclic structure that contributes to its aromatic properties.
Structurally, Limonene consists of a cyclic ring and an aliphatic chain, allowing effective interaction with olfactory receptors.
Physical Properties
Appearance: Typically a colorless or pale yellow liquid.
Solubility: Soluble in alcohol and oils; insoluble in water. It dissolves dammar and rosin. In the presence of inorganic acids, limonene reacts with water to form -terpinol and terpenediol hydrate.
pH: Neutral.
Odor: Fresh, citrusy, characteristic of citrus fruits.
Stability: Generally stable but sensitive to oxidation and light.
Production Process
Extraction: Limonene is extracted from citrus essential oils, such as lemon and orange, through distillation or cold pressing.
Purification: The product is purified to remove impurities and ensure quality.
Formulation: Purified Limonene is incorporated into various cosmetic and fragrance formulations.
Applications
Cosmetics: Commonly included in perfumes, lotions, and skin care products for its citrus scent.
INCI Functions:
Deodorant agent. When substances that give off an unpleasant odour are included in cosmetic formulations (typical examples are methyl mercaptan and hydrogen sulphide derived from garlic), deodorants attenuate or eliminate the unpleasant exhalation. It helps counteract the formation of bad odours on body surfaces.
Solvent. It is the substance for dissolving or dispersing surfactants, oils, dyes, flavourings, bactericidal preservatives in solution.In fact, it dissolves other components present in a cosmetic formulation. Solvents are generally liquid (aqueous and non-aqueous).
Perfuming. Unlike fragrance, which can also contain slightly less pleasant or characteristic odours, the term perfume indicates only very pleasant fragrances. Used for perfumes and aromatic raw materials.
Cosmetic safety
Restricted cosmetic ingredient as III/88 III/167 III/168 a Relevant Item in the Annexes of the European Cosmetics Regulation 1223/2009. Substance or ingredient reported:
The presence of the substance shall be indicated in the list of ingredients referred to in Article 19(1), point (g), when its concentration exceeds: — 0,001 % in leave-on products — 0,01 % in rinse-off products. The peroxide value for each substance shall be less than 20 mmoles/L (This limit applies to the substance and not to the finished cosmetic product).

Studies
Limonene has demonstrated a wide range of health benefits (1): has an anti-stress action (2), and recent studies have also focused on limonene as it could have a preventive anti-tumour action (3) due to its antioxidant activities, which act mainly on apoptosis induced by tumour regression (4). In this study, the antibacterial activity of d-limonene and its potentiating activity of different classes of antibiotics for Gram-positive and Gram-negative bacteria were positively evaluated (5).
Used as a natural solvent in the manufacture of oil and oil-based paints, synthetic resins, synthetic rubbers, metal drying agent and solvent.
Limonene is commonly used as a fragrance in cosmetics to pleasantly scent products containing it. It can be obtained from lemon, laurel, bergamot, kumquat, geranium and many other flowers. In cosmetic applications, as limonene is easily oxidised by contact with air, there is a risk of skin allergy (6).
Reading many cosmetic texts and reviews, limonene, like linalool, or linalool, are cited as dangerous components, but this is not the case. It is the oxidation of the product that can be potentially dangerous, not the component itself, as limonene, in cosmetics, is processed in a non-sterile way and in contact with components that can accelerate the oxidative process. This is why it is one of the chemicals that can cause allergies and why it is mandatory to write it on the label when its percentage exceeds a certain value. It is a component that is considered safe if it is not oxidised, so remember to close the cap of the package in which it is inserted tightly to prevent air from penetrating inside for a long time and causing oxidation not only of this, but also of any other oxidation-sensitive components.
Safety
Linalool and D-limonene are common fragrances that oxidise easily on exposure to air. The resulting linalool and D-limonene hydroperoxides have been shown to have high frequencies of positive patch test reactions in several European and international studies .... In this study, the frequency of positive patch test reactions to linalool hydroperoxides is 20% (19/96) and the frequency of positive reactions to D-limonene hydroperoxides is 8% (7/90). These high frequencies suggest that patch testing for linalool and limonene hydroperoxides should be performed in all patients with suspected fragrance allergy (7).
The most relevant studies on the subject have been selected with a summary of their contents:
Typical optimal characteristics of the commercial product d-limonene
| Appearance | Light Yellow Liquid |
| Boiling Point | 175.4±20.0 °C at 760 mmHg |
| Melting Point | -84--104 °C |
| Density D20g/cm3 | 0.841-0.860 |
| Index of Refraction | 1.472-1.485 |
| Flash Point | 42.8±0.0 °C |
| LogP | 4.45 |
| Vapour density | 4.7 |
| Vapour Pressure | 1.5±0.2 mmHg at 25°C |
| Content of Camphene ≤ | 1.0% |
 |  |
 |  |
- Molecular Formula : C10H16
- Molecular Weight : 136.238 g/mol
- Exact Mass 136.125198
- CAS : 138-86-3 9003-73-0 65996-98-7 7705-14-8 5989-27-5 8008-56-8 8008-57-9 8028-38-4 0008008-57-9
- UNII GFD7C86Q1W
- EC Number: 227-815-6 205-341-0 266-034-5 231-732-0
- DSSTox Substance ID: DTXSID6028288 DTXSID2029612
- MDL number MFCD00062992
- PubChem Substance ID
- InChI=1S/C10H16/c1-8(2)10-6-4-9(3)5-7-10/h4,10H,1,5-7H2,2-3H3
- InChl Key XMGQYMWWDOXHJM-UHFFFAOYSA-N
- SMILES CC1=CCC(CC1)C(=C)C
- IUPAC 1-methyl-4-prop-1-en-2-ylcyclohexene
- ChEBI 15384
- NSC 757069 21466 844
- RTECS GW6360000
- UN Number 2052
Synonyms :
- Dipentene
- Polylimonene
- Nesol
- Cyclohexene, 1-methyl-4-(1-methylethenyl)-, homopolymer
- 1-methyl-4-(prop-1-en-2-yl)cyclohex-1-ene
- p-Mentha-1,8-diene, polymers
- +)-p-Mentha-1,8-diene
- (+)-Carvene
- 4-Isopropenyl-1-methyl-1-cyclohexene
- EINECS 205-341-0
- EINECS 231-732-0
- MDL number: MFCD00062991
- PubChem Substance ID 329759707
References______________________________________________________________________
(1) Vieira AJ, Beserra FP, Souza MC, Totti BM, Rozza AL. Limonene: Aroma of innovation in health and disease. Chem Biol Interact. 2018 Mar 1;283:97-106. doi: 10.1016/j.cbi.2018.02.007.
(2) d'Alessio PA, Bisson JF, Béné MC. Anti-stress effects of d-limonene and its metabolite perillyl alcohol. Rejuvenation Res. 2014 Apr;17(2):145-9. doi: 10.1089/rej.2013.1515.
Abstract. Stress is closely linked by its biological mechanisms to inflammation and by its consequences to accelerated aging. Stress triggers a hormonal response along the hypothalamus-pituitary-adrenal (HPA) axis, which can disrupt the ortho/parasympathetic balance essential for a harmonious life. Proper nutrition, adequate physical activity, and limiting the harmful influence of stress play important roles in avoiding the development of disease and promoting healthy aging. d-Limonene, a monoterpene shown to reduce inflammatory parameters in several pre-clinical and clinical models, could also produce an anti-stress action by altering ortho/parasympathetic parameters as well as central neurotransmitter functions. Here we report on a rat model, where a functional observational battery (FOB) was performed by submitting animals to non-pathological stress. d-Limonene or its metabolite perillyl alcohol (POH) were administered per os at a dose of 10 mg/kg. FOB tests were performed 1 hr before gavage and then at 60, 120, and 180 min. These tests confirmed the stressed status of control rats fed vehicle. Conversely, a series of parameters were significantly less disturbed in treated rats, who retained a better activity and displayed less signs of stress. These effects were more pronounced and sustained after ingestion of d-limonene than POH, suggesting the role of endogeneous metabolization of the terpene. These studies show that d-limonene exerts, through its metabolite POH, a significant anti-stress action measurable by behavioral and physiologic parameters under the influence of the nervous system. In addition to its anti-inflammatory effects, a beneficial role as an anti-stress substance could thus be claimed for d-limonene used as a dietary supplement.
(3) Miller JA, Pappan K, Thompson PA, Want EJ, Siskos AP, Keun HC, Wulff J, Hu C, Lang JE, Chow HH. Plasma metabolomic profiles of breast cancer patients after short-term limonene intervention. Cancer Prev Res (Phila). 2015 Jan;8(1):86-93. doi: 10.1158/1940-6207.CAPR-14-0100.
da Silva CEH, Gosmann G, de Andrade SF. Limonene and Perillyl Alcohol Derivatives: Synthesis and Anticancer Activity. Mini Rev Med Chem. 2021;21(14):1813-1829. doi: 10.2174/1389557521666210212150504.
Abstract. Limonene and perillyl alcohol are natural monoterpenes that have attracted the attention of medicinal chemists due to their promising anticancer activities. Considering this, both compounds were explored as scaffolds to obtain various derivatives with anticancer activity. In this review, the data are organized for the first time, with a focus on the synthetic methods and strategies to obtain the derivatives throughout the period from 2000 to 2020. A brief discussion regarding the structure and activity relationships of the most active derivatives, stereoisomers, and their mechanisms of action is presented. Among the active compounds, a series of limonenes with thiosemicarbazone groups and perillyl alcohol hybrids with glycosides or drugs are illustrated. Taking all of this into account, this review may help researchers develop new promising anticancer candidates based on the structures of limonene and perillyl alcohol.
(4) de Vasconcelos C Braz J, de Carvalho FO, de Vasconcelos C Meneses D, Calixto FAF, Santana HSR, Almeida IB, de Aquino LAG, de Souza Araújo AA, Serafini MR. Mechanism of Action of Limonene in Tumor Cells: A Systematic Review and Meta-Analysis. Curr Pharm Des. 2021;27(26):2956-2965. doi: 10.2174/1381612826666201026152902.
(5) Costa MDS, Rocha JE, Campina FF, Silva ARP, Da Cruz RP, Pereira RLS, Quintans-Júnior LJ, De Menezes IRA, De S Araújo AA, De Freitas TS, Teixeira AMR, Coutinho HDM. Comparative analysis of the antibacterial and drug-modulatory effect of d-limonene alone and complexed with β-cyclodextrin. Eur J Pharm Sci. 2019 Feb 1;128:158-161. doi: 10.1016/j.ejps.2018.11.036.
(6) Deza G, García-Bravo B, Silvestre JF, Pastor-Nieto MA, González-Pérez R, Heras-Mendaza F, Mercader P, Fernández-Redondo V, Niklasson B, Giménez-Arnau AM; GEIDAC. Contact sensitization to limonene and linalool hydroperoxides in Spain: a GEIDAC* prospective study. Contact Dermatitis. 2017 Feb;76(2):74-80. doi: 10.1111/cod.12714.
(7) Nath NS, Liu B, Green C, Atwater AR. Contact Allergy to Hydroperoxides of Linalool and D-Limonene in a US Population. Dermatitis. 2017 Sep/Oct;28(5):313-316. doi: 10.1097/DER.0000000000000318.
Abstract. Background: Linalool and D-limonene are common fragrance ingredients that readily oxidize on exposure to air. The resulting hydroperoxides of linalool and D-limonene have been shown to have high frequencies of positive patch test reactions in several European and international studies. Objective: The aim of the study was to investigate the prevalence of contact allergy to the hydroperoxides of linalool and D-limonene in a US population. Methods: In this retrospective study, 103 patients with suspected fragrance allergy were patch tested to linalool 10% petrolatum (pet), hydroperoxides of linalool 1% pet, D-limonene 10% pet, and/or the hydroperoxides of D-limonene 0.3% pet between July 9, 2014, and October 25, 2016. Conclusions: In this study, the frequency of positive patch test reactions to the hydroperoxides of linalool is 20% (19/96), and the frequency of positive reactions to the hydroperoxides of D-limonene is 8% (7/90). These high frequencies suggest that patch testing to the hydroperoxides of linalool and limonene should be performed in all patients with suspected fragrance allergy.
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.EvaluateClose | (0 comments) |
Read other Tiiips about this object in __Italiano (2)
Component type: Chemical Main substances: Last update: 2021-12-20 09:58:18 | Chemical Risk: |