![]() PEG-5 Glyceryl Oleate
PEG-5 Glyceryl Oleate
Rating : 5.5
| Evaluation | N. Experts | Evaluation | N. Experts |
|---|---|---|---|
| 1 | 6 | ||
| 2 | 7 | ||
| 3 | 8 | ||
| 4 | 9 | ||
| 5 | 10 |
8 pts from Ark90
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.Evaluate | Where is this found? |
| "Glyceryl Oleate studies" about PEG-5 Glyceryl Oleate Review Consensus 10 by AColumn (9336 pt) | 2021-Dec-27 17:11 |
| Read the full Tiiip | (Send your comment) |
Compendium of the most significant studies with reference to properties, intake, effects.
Lian R, Lu Y, Qi J, Tan Y, Niu M, Guan P, Hu F, Wu W. Silymarin glyceryl monooleate/poloxamer 407 liquid crystalline matrices: physical characterization and enhanced oral bioavailability. AAPS PharmSciTech. 2011 Dec;12(4):1234-40. doi: 10.1208/s12249-011-9666-2.
Abstract. The main purpose of this study was to prepare silymarin glyceryl monooleate/poloxamer 407 liquid crystalline matrices (GMO/P407 LCM) to improve the oral bioavailability of silymarin.
Eskinazi-Budge A, Manickavasagam D, Czech T, Novak K, Kunzler J, Oyewumi MO. Preparation of emulsifying wax/glyceryl monooleate nanoparticles and evaluation as a delivery system for repurposing simvastatin in bone regeneration. Drug Dev Ind Pharm. 2018 Oct;44(10):1583-1590. doi: 10.1080/03639045.2018.1483381.
Abstract. In this study, we have investigated a new lipid nanoparticle (NP) platform that was fabricated using a binary blend of emulsifying wax (Ewax) and glyceryl monooleate (GMO).
Milak S, Zimmer A. Glycerol monooleate liquid crystalline phases used in drug delivery systems. Int J Pharm. 2015 Jan 30;478(2):569-87. doi: 10.1016/j.ijpharm.2014.11.072.
Abstract. This review presents recent progress in glycerol monooleate liquid crystalline phases used as drug delivery vehicles.
Sadhale Y, Shah JC. Glyceryl monooleate cubic phase gel as chemical stability enhancer of cefazolin and cefuroxime. Pharm Dev Technol. 1998 Nov;3(4):549-56. doi: 10.3109/10837459809028637.
Abstract. The primary objective of this study was to determine the ability of the glyceryl monooleate (GMO) cubic phase gel to protect drugs from chemical instability reactions such as hydrolysis and oxidation.
Ericsson EM, Faxälv L, Weissenrieder A, Askendal A, Lindahl TL, Tengvall P. Glycerol monooleate-blood interactions. Colloids Surf B Biointerfaces. 2009 Jan 1;68(1):20-6. doi: 10.1016/j.colsurfb.2008.09.016.
Abstract. In the present study the initial blood compatibility of glycerol monooleate (GMO)-coated surfaces was evaluated after deposition to surfaces and in bulk. The model surface was silica onto which multiple layers of fibrinogen or human serum albumin (HSA) was immobilized.
Nguyen TH, Hanley T, Porter CJ, Larson I, Boyd BJ. Phytantriol and glyceryl monooleate cubic liquid crystalline phases as sustained-release oral drug delivery systems for poorly water soluble drugs I. Phase behaviour in physiologically-relevant media. J Pharm Pharmacol. 2010 Jul;62(7):844-55. doi: 10.1211/jpp.62.06.0005.
Abstract. The potential utility of liquid crystalline lipid-based formulations in oral drug delivery is expected to depend critically on their structure formation and stability in gastrointestinal fluids. The phase behaviour of lipid-based liquid crystals formed by phytantriol and glyceryl monooleate, known to form a bicontinuous cubic phase in excess water, was therefore assessed in physiologically-relevant simulated gastrointestinal media.
Trickler WJ, Khurana J, Nagvekar AA, Dash AK. Chitosan and glyceryl monooleate nanostructures containing gemcitabine: potential delivery system for pancreatic cancer treatment. AAPS PharmSciTech. 2010 Mar;11(1):392-401. doi: 10.1208/s12249-010-9393-0.
Abstract. The objectives of this study are to enhance cellular accumulation of gemcitabine with chitosan/glyceryl monooleate (GMO) nanostructures, and to provide significant increase in cell death of human pancreatic cancer cells in vitro.
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.EvaluateClose | (0 comments) |
| "Descrizione" about PEG-5 Glyceryl Oleate Review Consensus 8 by Ark90 (12432 pt) | 2022-Nov-19 12:53 |
| Read the full Tiiip | (Send your comment) |
PEG-5 Glyceryl Oleate (Glyceryl monooleate) is an intermediate chemical compound prepared by the esterification of oleic acid. It contains glyceryl esters of fatty acids found in oleic acid. It appears as a transparent to straw yellow liquid.

The number after PEG represents the molecular weight and the higher the number, the less it penetrates the skin.
How it is produced
It is produced industrially by esterification of commercial oleic acid derived from edible sources or tall oil fatty acids. It contains and glyceryl esters of fatty acids found in commercial oleic acid.
What it is used for and where it is used
- Food: flavouring, solubilising, hydrophilic emulsifier.
- Cosmetics: antifoaming and dispersing agent. Stabilising emulsifier in oil/water formulations. Emulsifiers have the property of directly influencing the stability, sensory properties and surface tension of sunscreens by modulating their filmometric performance.
- Plastic: flux dropping and anti-fog agent, lubricant and antistatic agent.
- Textile: lubricant, emulsifier of industrial spinning oil.
Biodegradable and non-irritating.
This in vitro study finds that glyceryl monooleate (Glyceryl oleate) may play the role of a novel thermosensitive monoglyceride-based drug delivery system, particularly for local intracavitary chemotherapy (1).
This in vitro study evaluated the improvement of stratum corneum penetration kinetics by two common ingredients incorporated in topical skin formulations for skin protection and hydration, petrolatum and soybean oil. Glyceryl monooleate enhanced skin penetration for petrolatum (2).
Cubosomes are self-assembled liquid crystalline particles of certain surfactants and glyceryl monooleate is one of the most common surfactants used to produce cubosomes (3).
Safety
Glyceryl monooleate has been approved by the FDA (Food and Drug Administration) for food use and is considered biocompatible. It has been evaluated for genotoxicity, repeated dose toxicity, reproductive toxicity, local respiratory toxicity, phototoxicity/photoallergenicity, skin sensitisation and environmental safety. The data show that glyceryl monooleate is not genotoxic (4).
Glyceryl Oleate, Glyceryl monooleate studi
Typical optimal commercial product characteristics Glyceryl monooleate
| Appearance | Yellow to amber liquid |
| Boiling Point | 483.3±35.0 °C at 760 mmHg 409°C |
| Melting Point | 35-38ºC |
| Flash Point | 155.4±19.4 °C |
| Density | 0.9407 g/cm3 (35 ºC) |
| Acid value, mgKOH/g | 2.5 |
| Saponification value, mgKOH/g | 160~180 |
| Water content | ≤1.0 |
| Iodine value, gI2/100g ≤ | 113-123 |
| Fe, ppm ≤ | 20 |
| As, ppm ≤ | 5 |
| Heavy metals, ppm ≤ | 1 |
| PSA | 66.76000 |
| Pka | 13.16±0.20 |
| Solubility | chloroform: 50 mg/mL |
| LogP | 6.71 |
| Vapour Pressure | 0.0±2.8 mmHg at 25°C |
| Index of Refraction | 1.46384 (589.3 nm 35℃) |
| Storage | −20°C |
| Stability | Hygroscopic |
 |  |
 |  |
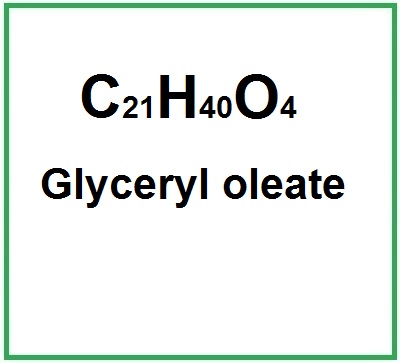
Molecular Formula : C21H40O4
- Molecular Weight : 356.5
- Exact Mass 356.292664
- CAS : 111-03-5 25496-72-4
- UNII D3AEF6S35P
- EC Number: 203-827-7 247-038-6 266-951-0
- DSSTox Substance ID: DTXSID6028319 DTXSID3027875 DTXSID3029360 DTXSID3042003
- MDL number MFCD00042735
- PubChem Substance ID 24897178
- IUPAC 2,3-dihydroxypropyl (Z)-octadec-9-enoate
- InChI=1S/C21H40O4/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-21(24)25-19-20(23)18-22/h9-10,20,22-23H,2-8,11-19H2,1H3/b10-9-
- InChl Key RZRNAYUHWVFMIP-KTKRTIGZSA-N
- SMILES CCCCCCCCC=CCCCCCCCC(=O)OCC(CO)O
- ChEBI 75342
- FEMA 2526
- NSC 406285
- JECFA 919
Synonyms:
- Glyceryl monooleate
- 1-Oleoyl-rac-glycerol
- 1-oleoylglycerol
- Glyceryl cis-9-octadecenoate
- DL-α-Monoolein
- Monoolein
- 1-(cis-9-Octadecenoyl)-rac-glycerol, rac-Glycerol 1-monooleate
References_________________________________________________________________
(1) Mengesha AE, Wydra RJ, Hilt JZ, Bummer PM. Binary blend of glyceryl monooleate and glyceryl monostearate for magnetically induced thermo-responsive local drug delivery system. Pharm Res. 2013 Dec;30(12):3214-24. doi: 10.1007/s11095-013-1230-1.
(2) Intarakumhaeng R, Shi Z, Wanasathop A, Stella QC, Wei KS, Styczynski PB, Li C, Smith ED, Li SK. In vitro skin penetration of petrolatum and soybean oil and effects of glyceryl monooleate. Int J Cosmet Sci. 2018 Aug;40(4):367-376. doi: 10.1111/ics.12469.
(3) Garg G, Saraf S, Saraf S. Cubosomes: an overview. Biol Pharm Bull. 2007 Feb;30(2):350-3. doi: 10.1248/bpb.30.350.
(4) Api AM, Belsito D, Biserta S, Botelho D, Bruze M, Burton GA Jr, Buschmann J, Cancellieri MA, Dagli ML, Date M, Dekant W, Deodhar C, Fryer AD, Gadhia S, Jones L, Joshi K, Kumar M, Lapczynski A, Lavelle M, Lee I, Liebler DC, Moustakas H, Na M, Penning TM, Ritacco G, Romine J, Sadekar N, Schultz TW, Selechnik D, Siddiqi F, Sipes IG, Sullivan G, Thakkar Y, Tokura Y. RIFM fragrance ingredient safety assessment, glyceryl monooleate, CAS Registry Number 111-03-5. Food Chem Toxicol. 2021 Mar;149 Suppl 1:111992. doi: 10.1016/j.fct.2021.111992.
___________________________
PEG stands for polyethylene glycols or polyethylene glycols, produced from water and condensed ethylene oxide, with various acts in medical, pharmaceutical, human food, animal feed, cosmetics and other fields. PEGs undergo thorough testing and specific safety assessment studies are required before they are placed on the market and are therefore generally considered safe. In the chemical production of PEG, impurities and production by-products such as ethylene oxide and 1,4-dioxane may be present, which are considered by the scientific literature to be known carcinogens. It is required by product control authorities that ethylene oxide and 1,4-dioxane are removed before they are mixed into formulations. Therefore, it is not correct to demonise the entire PEG category and try to avoid it as I see in some cosmetic product labels that flaunt 'PEG free', as the genotoxic hazard should be excluded upstream of the problem. At the very least, one would have to wonder if some companies might still be using non-certified PEGs, but the controls, at least in Europe and the United States, are quite strict.
And a premise on PEG.
Since the PEG (1) family is numerous and is found in many cosmetic, cleaning and medicinal products and others, we need a cognitive premise on the subject that is rather complex from the point of view of safety because these products not only come into contact with the skin but, as in the case of medicine, they are also ingested.
PEG or polyethylene glycols polymerise the condensed ethylene oxide and water and are called polyethylene glycols, but in reality, they are complex chemical components, polymers bound together. For example, plastic is polyethylene and has a hard consistency, while polyethylene aggregated to the glycol forms a liquid.
The number that appears after the initials PEG represents the molecular weight and the higher this number is, the less it penetrates the skin.
Here below are some studies in Medicine that refer to the use of PEG Polyethylene glycol in various fields.
Intestine
Polyethylene glycol with or without electrolytes is effective for the treatment of functional constipation, both in adults and in paediatric patients, with great safety and tolerability. These preparations are the most effective osmotic laxatives (more than lactulose) and are the first-line treatment for functional constipation in the short- and long-term. They are as effective as enemas in faecalomas, avoid the need for hospitalisation and are well tolerated by patients (especially when given without electrolytes) (2).
In the preparation for colonoscopy, polyethylene glycol tablets confirmed efficacy, acceptability, tolerance and safety similar to those of sodium phosphate (3).
For peripheral nerve repair (4).
Eyes
Dry eye syndrome is a disorder that affects 5-34% of the world's adult population with reduced quality of life. Artificial or lubricating tears are the most used therapy for treating this condition due to their low side effects profile, which attempt to modify the properties of the tear film. Polyethylene glycol has demonstrated clinical efficacy in the treatment of this condition (5).
Brain
Polyethylene glycol facilitates the neuroprotective effects of magnesium in head injuries (6).
Tumors
For transarterial chemoembolization, Polyethylene glycol is effective and safe for the treatment of liver cancer, as indicated by good tolerability, quality of life and high tumour response (7).
Cosmetics
Many types of PEG are hydrophilic and are used as creams, topical dermatological preparations and in cosmetic products such as surfactants, emulsifiers, detergents, humectants and skin conditioners.
Safety varies from type to type given the structural complexity (8).
References___________________________________________________________________
(1) Fruijtier-Pölloth C. Safety assessment on polyethylene glycols (PEGs) and their derivatives as used in cosmetic products. Toxicology. 2005 Oct 15;214(1-2):1-38. doi: 10.1016/j.tox.2005.06.001.
(2) Mínguez M, López Higueras A, Júdez J. Use of polyethylene glycol in functional constipation and fecal impaction. Rev Esp Enferm Dig. 2016 Dec;108(12):790-806. doi: 10.17235/reed.2016.4571/2016.
Santos-Jasso KA, Arredondo-García JL, Maza-Vallejos J, Lezama-Del Valle P. Effectiveness of senna vs polyethylene glycol as laxative therapy in children with constipation related to anorectal malformation. J Pediatr Surg. 2017 Jan;52(1):84-88. doi: 10.1016/j.jpedsurg.2016.10.021.
(3) Chaussade S, Schmöcker C, Toulemonde P, Muñoz-Navas M, O'Mahony V, Henri F. Phosphate tablets or polyethylene glycol for preparation to colonoscopy? A multicentre non-inferiority randomized controlled trial. Surg Endosc. 2017 May;31(5):2166-2173. doi: 10.1007/s00464-016-5214-1.
Tsunoda T, Sogo T, Iwasawa K, Umetsu S, Oikawa-Kawamoto M, Inui A, Fujisawa T. Feasibility and safety of bowel cleansing using low-volume polyethylene glycol with ascorbic acid before pediatric colonoscopy: A pilot study. Dig Endosc. 2017 Mar;29(2):160-167. doi: 10.1111/den.12756.
(4) Hoffman AN, Bamba R, Pollins AC, Thayer WP. Analysis of polyethylene glycol (PEG) fusion in cultured neuroblastoma cells via flow cytometry: Techniques & optimization. J Clin Neurosci. 2017 Feb;36:125-128. doi: 10.1016/j.jocn.2016.10.032.
(5) Pérez-Balbuena AL, Ochoa-Tabares JC, Belalcazar-Rey S, Urzúa-Salinas C, Saucedo-Rodríguez LR, Velasco-Ramos R, Suárez-Sánchez RG, Rodríguez-Carrizalez AD, Oregón-Miranda AA. Efficacy of a fixed combination of 0.09 % xanthan gum/0.1 % chondroitin sulfate preservative free vs polyethylene glycol/propylene glycol in subjects with dry eye disease: a multicenter randomized controlled trial. BMC Ophthalmol. 2016 Sep 20;16(1):164. doi: 10.1186/s12886-016-0343-9.
Labetoulle M, Messmer EM, Pisella PJ, Ogundele A, Baudouin C. Safety and efficacy of a hydroxypropyl guar/polyethylene glycol/propylene glycol-based lubricant eye-drop in patients with dry eye. Br J Ophthalmol. 2017 Apr;101(4):487-492. doi: 10.1136/bjophthalmol-2016-308608.
(6) Busingye DS, Turner RJ, Vink R. Combined Magnesium/Polyethylene Glycol Facilitates the Neuroprotective Effects of Magnesium in Traumatic Brain Injury at a Reduced Magnesium Dose. CNS Neurosci Ther. 2016 Oct;22(10):854-9. doi: 10.1111/cns.12591.
(7) Aliberti C, Carandina R, Sarti D, Mulazzani L, Catalano V, Felicioli A, Coschiera P, Fiorentini G. Hepatic Arterial Infusion of Polyethylene Glycol Drug-eluting Beads for Primary and Metastatic Liver Cancer Therapy. Anticancer Res. 2016 Jul;36(7):3515-21.
(8) Jang HJ, Shin CY, Kim KB. Safety Evaluation of Polyethylene Glycol (PEG) Compounds for Cosmetic Use. Toxicol Res. 2015 Jun;31(2):105-36. doi: 10.5487/TR.2015.31.2.105.
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.EvaluateClose | (0 comments) |
Read other Tiiips about this object in __Italiano (2)
Component type: Natural Main substances: Last update: 2018-02-19 18:45:58 | Chemical Risk: |