![]() Cocamidopropyl betaine
Cocamidopropyl betaine
Rating : 6
| Evaluation | N. Experts | Evaluation | N. Experts |
|---|---|---|---|
| 1 | 6 | ||
| 2 | 7 | ||
| 3 | 8 | ||
| 4 | 9 | ||
| 5 | 10 |
Cons:
Specific allergy (1)10 pts from Ark90
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.Evaluate | Where is this found? |
| "Cocamidopropyl betaine studies" about Cocamidopropyl betaine Review Consensus 7 by Al222 (20707 pt) | 2021-Dec-25 19:19 |
| Read the full Tiiip | (Send your comment) |
Irritation from surfactants in detergents is a rather frequent reaction in the use of cosmetics and sensitisation to the product is also possible. Cocamidopropyl betaine in the Italian standard (Società Italiana di Dermatologia Allergologica, Professionale e Ambientale [SIDAPA]) gave five positive reactions among 105 patients in a scientific test (1).
Other studies have shown allergies, but to a lesser extent (2).
The Cosmetic Ingredient Review expert group concluded in 2012 that this chemical compound did not present any significant toxicity and was therefore safe for use as a cosmetic ingredient (3).
References____________________________________________________
(1) Corazza M, Lauriola MM, Bianchi A, Zappaterra M, Virgili A. Irritant and sensitizing potential of eight surfactants commonly used in skin cleansers: an evaluation of 105 patients. Dermatitis. 2010 Sep-Oct;21(5):262-8.
(2) Mertens S, Gilissen L, Goossens A. Allergic contact dermatitis caused by cocamide diethanolamine. Contact Dermatitis. 2016 Jul;75(1):20-4. doi: 10.1111/cod.12580.
(3) Burnett CL, Bergfeld WF, Belsito DV, Hill RA, Klaassen CD, Liebler D, Marks JG Jr, Shank RC, Slaga TJ, Snyder PW, Andersen FA. Final report of the Cosmetic Ingredient Review Expert Panel on the safety assessment of cocamidopropyl betaine (CAPB). Int J Toxicol. 2012 Jul-Aug;31(4 Suppl):77S-111S. doi: 10.1177/1091581812447202.
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.EvaluateClose | (0 comments) |
| "Descrizione" about Cocamidopropyl betaine Review Consensus 10 by Ark90 (12432 pt) | 2024-Oct-06 11:29 |
| Read the full Tiiip | (Send your comment) |
Cocamidopropyl betaine is a pseudo-amphoteric chemical compound, zwitterionic, with a quaternary ammonium cation that is industrially produced from coconut oil and dimethylaminopropylamine.
Cocamidopropyl Betaine (CAPB) is a synthetic surfactant derived from coconut oil, commonly used in personal care products, household cleaners, and industrial applications. It is an amphoteric compound, meaning it has both positive and negative charges, which allows it to act as a mild detergent, foam booster, and conditioning agent. It is found in products such as shampoos, body washes, and liquid soaps due to its ability to enhance foam, cleanse gently, and reduce irritation compared to harsher surfactants.
Chemical Composition and Structure
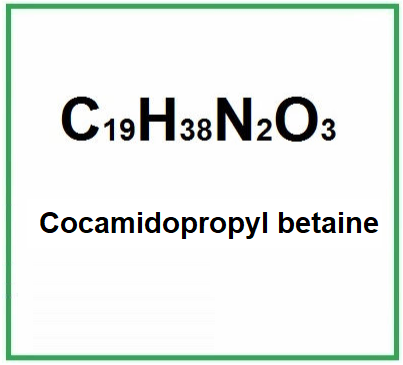
Cocamidopropyl Betaine is made from the fatty acids of coconut oil, primarily lauric acid, which is reacted with 3-dimethylaminopropylamine to form the amide structure. The resulting product is further reacted with chloroacetic acid to produce the betaine portion. Its chemical formula is C19H38N2O3, and the compound contains both a hydrophilic (water-attracting) and hydrophobic (water-repelling) part, which allows it to act as an effective surfactant.
Physical Properties
Cocamidopropyl Betaine is typically found as a yellow to pale amber liquid. It is soluble in water, allowing it to mix well with other ingredients in formulations. CAPB is known for being a mild surfactant that helps boost foam formation, making it ideal for use in products that require a rich lather, such as shampoos and body washes.
The name describes the structure of the molecule:
Cocamidopropyl is a compound derived from coconut oil and dimethylaminopropylamine. It contains a long chain of carbon atoms (from coconut oil fatty acids), a nitrogen atom (N) and three hydrogen atoms forming an amine group (-NH2).
betaine is a compound known as zwitterion, which has positive and negative charges but is neutral overall. In Cocamidopropyl betaine, the betaine part of the molecule is derived from the cocamidopropyl part by replacing one of the hydrogen atoms of the amine group with a carboxyl group (-COO-).
The synthesis process takes place in different steps:
- Extraction. The raw materials are coconut oil and betaine. Coconut oil is extracted from ripe coconuts, while betaine is usually obtained from sugar beets.
- Cocamide production. Coconut oil fatty acids are isolated and then reacted with dimethylaminopropylamine to produce cocamidopropylamine.
- Reaction. Cocamidopropylamine is reacted with betaine and an acid catalyst to produce cocamidopropyl betaine.
- Purification. The resulting product, in a series of steps that may include distillation and filtration, is purified to remove all unreacted materials and by-products.
- Quality control. The final product is tested to ensure that it meets quality standards.
It occurs as a clear to pale yellow transparent liquid or as a fine white powder. It dissolves easily in water (10 % solution), has an acid reaction with a pH of 5-7.

What it is used for and where it is used
Cosmetics
It is a surfactant (removes dirt particles) of synthetic origin and is used in cosmetics and body cleansers with an antimicrobial and foaming function. Softens hair and reduces static electricity in conditioners. Thickener in personal care products and detergents. Improves the conditioning functions of other surfactants, performs well against water hardness, is antistatic and biodegradable. Good compatibility with other amphoteric surfactants and cationic, anionic, non-ionic surfactants.
Cocamidopropyl betaine is one of the most common chemical compounds used in cosmetics and personal hygiene in detergents, liquid soaps, shampoos, eye make-up products, make-up removers, bath gels, contact lens solutions, roll-on deodorants. In shampoos the recommended dosage is 3-9%, while in cosmetics 1-2% is sufficient.
It has a significant number of INCI functions:
- Antistatic agent. Static electricity build-up has a direct influence on products and causes electrostatic adsorption. The antistatic ingredient reduces static build-up and surface resistivity on the surface of the skin and hair.
- Cleansing agent. Ingredient that cleanses skin without exploiting the surface-active properties that produce a lowering of the surface tension of the stratum corneum.
- Hair conditioning agent. A large number of ingredients with specific purposes can co-exist in a hair shampoo: cleansers, conditioners, thickeners, mattifying agents, sequestering agents, fragrances, preservatives, special additives. However, the indispensable ingredients are the cleansers and conditioners as they are necessary and sufficient for hair cleansing and manageability. The others act as commercial and non-essential auxiliaries such as: appearance, fragrance, colouring, etc. Hair conditioning agents have the task of increasing shine, manageability and volume, and reducing static electricity, especially after treatments such as colouring, ironing, waving, drying and brushing. They are, in practice, dispersing agents that may contain cationic surfactants, thickeners, emollients, polymers. The typology of hair conditioners includes: intensive conditioners, instant conditioners, thickening conditioners, drying conditioners.
- Skin conditioning agent - Miscellaneous. This ingredient has the task of modifying the condition of the skin when it is damaged or dry by reducing its flakiness and restoring its elasticity.
- Surfactant - Cleansing agent. Cosmetic products used to cleanse the skin utilise the surface-active action that produces a lowering of the surface tension of the stratum corneum, facilitating the removal of dirt and impurities.
- Surfactant - Foam booster. It has the effect of introducing gas bubbles into the water and affects the cleaning process by helping to spread the cleanser. Since sebum has an inhibiting effect on the bubble, more foam is produced in the second shampoo.
- Viscosity control agent. It controls and adapts viscosity to the required level for optimal chemical and physical stability of the product and dosage in gels, suspensions, emulsions, solutions.
However, the presence of salt in solutions containing Cocamidopropyl betaine reduces their ability to lower surface tension, decreases the critical micelle concentration (mol/dm3) and increases absorption parameters (1).
Medical
In medicine and pharmaceuticals it is used in preparations for treating acne, exfoliating and peel-off products, anti-dandruff products etc..
Other uses
- In the detergent industry it is in hand washing detergents, hand dishwashing products.
- In the pesticide industry as a surfactant instead of alkyl polyglycosides (APG) and polyoxyethylene amine surfactants (TAE), which have proven to be quite toxic to the environment and irritating to the human epidermis.
- In the textile industry as a softening agent.
The objectives of this in vitro study were: a) to determine the effects of the waiting period of chlorhexidine (CHX) rinse after the use of fluoride toothpaste and b) to further determine the effect of the surfactant in the toothpaste [sodium dodecyl sulfate (SDS) or Cocamidopropyl betaine (CAPB)] on the remineralisation of the caries lesion associated with CHX rinse. The absence of CHX as an adjunct to fluoride toothpastes resulted in greater remineralisation of enamel lesions than the immediate use of CHX treatment for toothpastes with SDS and CAPB. CAPB toothpastes indicated significantly greater remineralisation than SDS toothpastes and may be recommended for patients at high risk of caries. A waiting time of 30 minutes for CHX treatment is recommended after brushing (2).
Safety
Cocamidopropyl betaine is normally among the least allergenic preservative chemical compounds, however its relative allergenicity appears to be attributed to its impurities dimethylaminopropylamine and cocamidopropyl dimethylamine and typically manifests as hand dermatitis (3).
Repeated and prolonged use of surfactants can cause irritation and allergic contact dermatitis. (4).
The results of this study are discussed in terms of the environmental consequences of the application of CAPB to the control of harmful blooms on algae (5).
Cocamidopropyl betaine (CAPB) and related amidopropylbainins are zwitterions mainly used as surfactants in cosmetics. These ingredients are safe for use as cosmetic ingredients in the use and concentration practices of this safety assessment (6).
The most relevant studies on the subject have been selected with a summary of their contents:
Cocamidopropyl betaine studies
Typical optimal commercial product characteristics Cocamidopropyl betaine
| Appearance | Light yellow clear liquid |
| Melting point | < −10 °C (14 °F; 263 K) -50°C |
| Boiling point | 100°C 120°C |
| Flash point | 94°C |
| Solid Content | 35% ±1% 40% 45% |
| Free amine content | 0.5% max |
| Solid content | 35.0% min. |
| Active matter | 28.0% min. |
| pH value (5% aq.solution, 25℃) | 5-7 |
| Sodium chloride content | 7.0% max |
| PSA | 121.27000 |
| Free monochloroacetic acid | Max.100ppm |
| Sodium chloride | 6.0-7.0% Max |
| Free amine | 0.5% Max |
| Spec.gravity at 20℃ | 1.045-1.070 |
 |  |
 |  |
- Molecular Formula C19H38N2O3 RCONH(CH2)3N+(CH3)2CH2COO
- Molecular weight 342.524 g/mol
- Exact Mass 344.27874102
- CAS : 61789-40-0 86438-79-1 4292-10-8
- UNII 23D6XVI233
- EC Number 263-058-8
- DSSTox Substance ID DTXSID6028072 DTXSID4041282
- MDL number MFCD00239947
- PubChem Substance ID
- IUPAC 2-[3-(dodecanoylamino)propyl-dimethylazaniumyl]acetate
- InChI=1S/C19H38N2O3/c1-4-5-6-7-8-9-10-11-12-14-18(22)20-15-13-16-21(2,3)17-19(23)24/h4-17H2,1-3H3,(H-,20,22,23,24)
- InChl Key MRUAUOIMASANKQ-UHFFFAOYSA-N
- SMILES CCCCCCCCCCCC(=O)NCCC[N+](C)(C)CC(=O)[O-]
- SCHEMBL 22684
- NSC 8191
Synonyms :
- Lauroylamide propylbetaine
- EINECS 224-292-6
- Dimethyl(lauramidopropyl)betaine
- Lauroylaminopropyldimethylaminoacetate
- N-Laurylamidopropyl-N,N-dimethylbetaine
- 3-Lauroylamidopropyl betaine
- [3-(Lauroylamino)propyl]dimethylaminoacetic acid
- (3-(Lauroylamino)propyl)dimethylaminoacetic acid
- Cocoamphocarboxypropionate
- 1-Propanaminium, N-(carboxymethyl)-N,N-dimethyl-3-((1-oxododecyl)amino)-, inner salt
- Lauroylamidopropylbetaine
- 1-Propanaminium, N-(carboxymethyl)-N,N-dimethyl- 3-[(1-oxododecyl)amino]-, hydroxide, inner salt
- (3-Laurylaminopropyl)dimethylaminoacetic acid, hydroxide, inner salt
- (3-Lauramidopropyl)dimethylbetaine
- N,N-Dimethyl-N-dodecanoylaminopropylbetaine
- 2-[(3-Dodecanamidopropyl)dimethylaminio]acetate
- N-(Dodecylamidopropyl)-N,N-dimethylammonium betaine
- 2-[3-(dodecanoylamino)propyl-dimethylazaniumyl]acetate
- {[3-(Dodecanoylamino)propyl](dimethyl)ammonio}acetate
- (3-Laurylaminopropyl)dimethylaminoacetic acid, inner salt
- 1-Propanaminium, N-(carboxymethyl)-N,N-dimethyl-3-((1-oxododecyl)amino)-, hydroxide, inner salt
- N-(Carboxymethyl)-N,N-dimethyl-3-[(1-oxododecyl)amino]-1-propanaminium Hydroxide Inner Salt
- Ammonium, (carboxymethyl)(3-lauramidopropyl)dimethyl-, hydroxide, inner salt
- (Carboxymethyl)(3-lauramidopropyl)dimethylammonium Hydroxide Inner Salt
- 1-Propanaminium,N-dimethyl-3-[(1-oxododecyl)amino]-, hydroxide, inner salt
- beta-Alanine, N-(2-aminoethyl)-N-(2-(2-carboxyethoxy)ethyl)-, norcoco acyl derivs., disodium salts
- N-(2-Aminoethyl)-N-(2-(2-carboxyethoxy)ethyl) beta-alanine, norcoco acyl derivs., disodium salts
- N-(Carboxymethyl)-N,N-dimethyl-3-((1-oxococonut)amino)-1-propanam- inium hydroxide, inner salt
- N-(Carboxymethyl)-N,N-dimethyl-3-((1-oxododecyl)amino)-1-propanam- inium hydroxide, inner salt
- Quaternary ammonium compounds, (carboxymethyl)(3-cocoamidopropyl)dimethyl, hydroxides, inner salts
References_______________________________________________________________________
(1) Staszak K, Wieczorek D, Michocka K. Effect of Sodium Chloride on the Surface and Wetting Properties of Aqueous Solutions of Cocamidopropyl Betaine. J Surfactants Deterg. 2015;18(2):321-328. doi: 10.1007/s11743-014-1644-8.
Abstract. Surfactants are important ingredients of personal care products and household products. The main characteristic of these compounds is to decrease the surface tension of solvent and resulting many properties such as contact angle, foam properties etc. The coexistence of other ingredients in the product may affect the properties of surfactants. One of the main components contained in almost every personal care and household product is sodium chloride. The main aim of this work was to determine the effect of this salt on some surface and usage properties of cocamidopropyl betaine (CAPB). From our experiments it was shown that the effect of added sodium chloride in the aqueous solutions of CAPB on the properties is the opposite to the one described in the literature for cationic and anionic surfactants, i.e., CMC increases with increasing ionic strength, foam height decreases with increasing salt concentration. Our investigation showed that sodium chloride makes worse the properties of the CAPB solutions examined in this work.
(2) Almohefer SA, Levon JA, Gregory RL, Eckert GJ, Lippert F. Caries lesion remineralization with fluoride toothpastes and chlorhexidine - effects of application timing and toothpaste surfactant. J Appl Oral Sci. 2018 Jun 11;26:e20170499. doi: 10.1590/1678-7757-2017-0499.
Abstract. Introduction: Habitual toothbrushing with fluoridated toothpaste followed by rinsing with antibacterial mouthwashes is a method to maintain good oral hygiene and to diminish the occurrence and severity of dental caries and periodontal disease. However, our understanding of how antimicrobial agents in mouthwashes affect fluoride-mediated caries lesion remineralization is still poor. Objective: The objectives of this in vitro study were a) to determine the effects of the waiting period of chlorhexidine (CHX) rinsing after fluoride toothpaste use and b) to further determine the effect of the type of toothpaste surfactant [sodium dodecyl sulfate (SDS) or cocamidopropyl betaine (CAPB)] on caries lesion remineralization associated with CHX rinsing....Conclusions: The absence of CHX as an adjunct to fluoride toothpastes led to greater remineralization of enamel lesions compared with the immediate use of CHX treatment for both SDS- and CAPB-toothpastes. CAPB-toothpastes indicated significantly greater remineralization than SDS-toothpastes, and can be suggested for patients at high risk of caries. A 30-minute waiting time for CHX treatment is recommended after brushing.
(3) Suuronen K, Pesonen M, Aalto-Korte K. Occupational contact allergy to cocamidopropyl betaine and its impurities. Contact Dermatitis. 2012 May;66(5):286-92. doi: 10.1111/j.1600-0536.2011.02036.x.
(4) Fowler JF Jr, Shaughnessy CN, Belsito DV, DeKoven JG, Deleo VA, Fransway AF, Maibach HI, Marks JG, Mathias CG, Pratt M, Sasseville D, Taylor JS, Warshaw EM, Zirwas MJ, Zug KA, Lorenz D. Cutaneous Delayed-Type Hypersensitivity to Surfactants. Dermatitis. 2015 Nov-Dec;26(6):268-70. doi: 10.1097/DER.0000000000000150.
Abstract. Background: Repeated and prolonged use of surfactants can cause irritant as well as allergic contact dermatitis. Objective: This study reports the frequency of positive patch test results to surfactants tested on the North American Contact Dermatitis Group screening series including cocamidopropyl betaine (CAPB), amidoamine (AA), dimethylaminopropylamine (DMAPA), oleamidopropyl dimethylamine (OPD), and cocamide diethanolamide (CDEA), and correlations of positive reactions between CAPB and the other surfactants.....Conclusions: The OPD had the highest rate of positive patch reactions (2.3%) followed by DMAPA (1.7%), and CAPB (1.4%). The AA and CDEA had the lowest rate of positive reactions (0.8%). There was a high degree of overlap in positive patch tests between the surfactants. The CDEA was the least likely to coreact with another surfactant.
(5) Vonlanthen S, Brown MT, Turner A. Toxicity of the amphoteric surfactant, cocamidopropyl betaine, to the marine macroalga, Ulva lactuca. Ecotoxicology. 2011 Jan;20(1):202-7. doi: 10.1007/s10646-010-0571-3.
Abstract. The degradation of the synthetic, amphoteric surfactant, cocamidopropyl betaine (CAPB) and its toxicity to the marine macroalga, Ulva lactuca, has been evaluated using several different physiological test end-points over different periods of exposure up to 120 h. Droplet surface angle measurements revealed that, following a period of acclimation of about 24 h, CAPB began to degrade and that primary degradation was complete within 120 h. Effective quantum yield (∆F/F(m)') and relative growth rates (RGRs) were the most sensitive measures of phytotoxicity, with CAPB concentrations at and above 10 mg l(-1) eliciting irreversible, time-dependent and/or dose-dependent responses. Cell membrane damage, estimated from measurements of ion leakage, was detected only at a concentration of 40 mg l(-1) after 48 h of exposure to CAPB but by 120 h damage was evident at all measured concentrations above 10 mg l(-1). These observations suggest that both CAPB and its metabolites are intrinsically toxic to U. lactuca. The findings of this study are discussed in terms of the environmental consequences of applying CAPB to control harmful algal blooms.
(6) Burnett CL, Bergfeld WF, Belsito DV, Hill RA, Klaassen CD, Liebler D, Marks JG Jr, Shank RC, Slaga TJ, Snyder PW, Andersen FA. Final report of the Cosmetic Ingredient Review Expert Panel on the safety assessment of cocamidopropyl betaine (CAPB). Int J Toxicol. 2012 Jul-Aug;31(4 Suppl):77S-111S. doi: 10.1177/1091581812447202.
Abstract Cocamidopropyl betaine (CAPB) and related amidopropyl betaines are zwitterions used mainly as surfactants in cosmetics. These cosmetic ingredients are similar in their chemistry, in particular with respect to the presence of 3,3-dimethylamino-propylamine (DMAPA) and fatty acid amidopropyl dimethylamine (amidoamine) impurities, which are known as sensitizers. The CIR Expert Panel concluded that because these ingredients present no other significant toxicity, when formulated to be nonsensitizing (which may be based on a quantitative risk assessment), these ingredients are safe for use as cosmetic ingredients in the practices of use and concentration of this safety assessment.
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.EvaluateClose | (0 comments) |
Read other Tiiips about this object in __Italiano (2)
Component type: Chemical Main substances:
Last update: 2021-12-24 19:32:10 | Chemical Risk: |