Check the ingredients!
... live healthy!


![]() PEG-40 Hydrogenated Castor Oil
PEG-40 Hydrogenated Castor Oil
Rating : 6.3
| Evaluation | N. Experts | Evaluation | N. Experts |
|---|---|---|---|
| 1 | 6 | ||
| 2 | 7 | ||
| 3 | 8 | ||
| 4 | 9 | ||
| 5 | 10 |
18 pts from Al222
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.Evaluate | Where is this found? |
| "PEG-40 Hydrogenated Castor Oil stu" about PEG-40 Hydrogenated Castor Oil Review Consensus 7 by Al222 (19780 pt) | 2022-Oct-16 11:19 |
| Read the full Tiiip | (Send your comment) |
Compendium of the most significant studies with reference to properties, intake, effects.
Rachmawati H, Novel MA, Ayu S, Berlian G, Tandrasasmita OM, Tjandrawinata RR, Anggadiredja K. The In Vitro-In Vivo Safety Confirmation of PEG-40 Hydrogenated Castor Oil as a Surfactant for Oral Nanoemulsion Formulation. Sci Pharm. 2017 Mar 31;85(2):18. doi: 10.3390/scipharm85020018.
Abstract. Evaluation on the safety use of high concentration of polyoxyl 40 (PEG-40) hydrogenated castor oil as a surfactant for oral nanoemulsion was performed in Webster mice. As previously reported, nearly 20% of PEG-40 hydrogenated castor oil was used to emulsify the glyceryl monooleate (GMO) as an oil to the aqueous phase. Thermodynamically stable and spontaneous nanoemulsion was formed by the presence of co-surfactant polyethylene glycol 400 (PEG-400). Standard parameters were analyzed for nanoemulsion including particle size and particle size distribution, the surface charge of nanoemulsion, and morphology. To ensure the safety of this nanoemulsion, several cell lines were used for cytotoxicity study. In addition, 5000 mg/kg body weight (BW) of the blank nanoemulsion was given orally to Webster mice once a day for 14 days. Several parameters such as gross anatomy, body weight, and main organs histopathology were observed. In particular, by considering the in vivo data, it is suggested that nanoemulsion composed with a high amount of PEG-40 hydrogenated castor oil is acceptable for oral delivery of active compounds.
Djekic L, Ibric S, Primorac M. The application of artificial neural networks in the prediction of microemulsion phase boundaries in PEG-8 caprylic/capric glycerides based systems. Int J Pharm. 2008 Sep 1;361(1-2):41-6. doi: 10.1016/j.ijpharm.2008.05.002.
Abstract. The objective of this study was to develop artificial neural network (ANN) model suitable to predict successfully the borders of the microemulsion region in the quaternary system PEG-8 caprylic/capric glycerides (Labrasol)/cosurfactant/isopropyl myristate/water, in order to minimise experimental effort. In our preliminary investigations of phase behaviour, two cosurfactants were used, PEG-40 hydrogenated castor oil (Cremophor) RH 40) and polyglyceryl-6 isostearate (Plurol Isostearique). Microemulsion existance area in pseudo-ternary phase diagrams was determined using titration method at constant: (a) oil-to-water ratio (alpha=50%, w/w); (b) surfactant-to-cosurfactant ratio (Km) 4:6; (c) Km 5:5; or (d) Km 6:4. It was found that the phase behaviour of systems involving polyoxyethylene type of cosurfactant depends significantly on oil-to-surfactant/cosurfactant mixture mass ratio (O/SCoS) but it is Km-independent. The formation of microemulsions in Labrasol/polyglyceryl-6 isostearate based systems was a complex function of Km and O/SCoS and there was employed a Generalized Regression Neural Network (GRNN) with four layers as a predictive mathematical model, using data obtained from the phase behaviour study (the surfactant concentration in surfactant/cosurfactant mixture (S, %, w/w), the oil concentration in the mixture with tensides (O, %, w/w) as two input variables, and the water solubilization limit (W(max), %, w/w) as output data). After network training, six independent pairs of input/output data were used for network testing. The resulting GRNN was tested statistically and found to be of quality predictive power. This results confirmed that the trained GRNN could be effective in predicting the size of the microemulsion area providing valuable tool in formulation of this type of colloidal vehicles.
Deyab MA. Utilization of a nonionic surfactant for improved corrosion resistance of carbon steel in simulated fuel-grade ethanol. RSC Adv. 2018 Jun 7;8(37):20996-21001. doi: 10.1039/c8ra02936a.
Abstract. In this study, a nonionic surfactant (PEG-40 hydrogenated castor oil, Abbrev. PEG-40 HCO) was used to improve the corrosion resistance of carbon steel in simulated fuel-grade ethanol (SFGE). The studies were conducted using cyclic voltammetry (CV) and potentiodynamic polarization techniques and complemented by scanning electron microscopy (SEM) investigations. The presence of water and chloride ions in SFGE strongly influences the electrochemical behavior of carbon steel. Polarization curves indicate that PEG-40 HCO has good inhibition efficiency and behaves as a mixed inhibitor. The inhibition efficiency increases with the concentration of PEG-40 HCO within the range of 20 to 100 ppm, reaching a maximum value of 93.8%. The adsorption of PEG-40 HCO obeys the Langmuir adsorption isotherm. Quantum chemical calculations were evaluated to confirm experimental results. This journal is © The Royal Society of Chemistry.
Hua L, Weisan P, Jiayu L, Hongfei L. Preparation and evaluation of microemulsion of vinpocetine for transdermal delivery. Pharmazie. 2004 Apr;59(4):274-8.
Abstract. Poorly soluble vinpocetine was selected as the model drug to prepare a microemulsion in order to increase solubility and in vitro transdermal delivery of the drug. Oleic acid was chosen as the oil phase due to its excellent solubilizing capacity. PEG-40 hydrogenated castor oil (Cremophor RH40) was employed as a surfactant (S) and purified diethylene glycol monoethyl ether (Transcutol P) was used as a cosurfactant (CoS). The effects of diverse types of oil, different weight ratios of surfactant to cosurfactant (S/CoS) on the solubility and permeation rate of vinpocetine were investigated. The optimized microemulsion consisted of 1% vinpocetine, 4% oleic acid, 20% Cremophor RH40, 10% Transcutol P and 65% distilled water (w/w), in which drug solubility was about 2,100 fold compared to that in water and the apparent permeation rate across the excised rat skin was 15.0 +/- 2.5 microg/cm2/h. Finally the physicochemical properties of the optimized microemulsion including pH, viscosity, refractive index, conductivity and particle size distribution were examined, which showed stable behavior after more than 12 months at ambient temperature. The irritation study showed that optimized microemulsion was a safe transdermal delivery system.
Milović M, Djuriš J, Djekić L, Vasiljević D, Ibrić S. Characterization and evaluation of solid self-microemulsifying drug delivery systems with porous carriers as systems for improved carbamazepine release. Int J Pharm. 2012 Oct 15;436(1-2):58-65. doi: 10.1016/j.ijpharm.2012.06.032.
Abstract. The purpose of this study was to investigate solid self-microemulsifying drug delivery system (SSMEDDS), as potential delivery system for poorly water soluble drug carbamazepine (CBZ)....Copyright © 2012 Elsevier B.V.
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.EvaluateClose | (0 comments) |
| "Descrizione" about PEG-40 Hydrogenated Castor Oil Review Consensus 18 by Al222 (19780 pt) | 2023-Nov-26 12:12 |
| Read the full Tiiip | (Send your comment) |
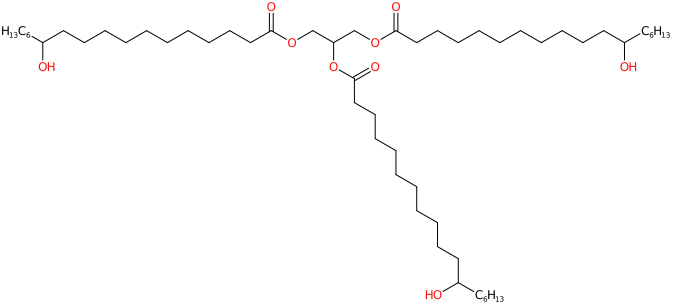
PEG-40 Hydrogenated Castor Oil is a brittle and hard vegetable wax. a polyethylene glycol derivative of castor oil. It is used as an emulsifier and surfactant in various cosmetic and personal care products. Its primary function is to help mix water with oils and other ingredients that would not normally blend together.
The name defines the structure of the molecule:
The synthesis process takes place in several stages:
So,a combination of synthetic polyethylene glycol (PEG) obtained by catalytic hydrogenation of chemically produced castor oil using an ethoxylation process with a chemical reaction in which ethylene oxide is added to a castor oil substrate. It is mixed through etherification and esterification of hydrogenated castor oil glycerides and fatty acids with forty ethylene oxide units. The number 40 appearing after the abbreviation PEG represents the molecular weight and the higher this number is, the less it penetrates the skin.
Synthetically, it appears as white flakes or an oily transparent liquid. Flammable. Incompatible with strong oxidising agents. Insoluble in water and characteristic smell. It contains 40 mol of ethylene oxide.


What it is used for and where
Hydrogenated castor oil is used in water to solubilise synthetic spices and essential oils by mixing the required solubilising substance in the proportion of 1: 1-3 while stirring until a transparent mixture is achieved.
Cosmetics
Non-ionic surfactant, rheological additive for sunscreens, production and manufacture of various water-based products for washing, make-up, rich in omega fatty acids possesses antifungal and antibacterial activity. It is an emulsifier and moisturiser used in cosmetic formulations for liquid soaps, shampoos, tanning creams and more. Useful in the treatment of dandruff and as a conditioner for dry hair.
Emulsion stabiliser. Emulsions are thermodynamically unstable. Emulsion stabilisers improve the formation and stability of single and double emulsions. as well as their shelf-life. It should be noted that in the structure-function relationship, the molar mass of the ingredient used plays an important role.
Surfactant - Emulsifying agent. Emulsions are thermodynamically unstable and are used to soothe or soften the skin and emulsify, so they need a specific, stabilising ingredient. This ingredient forms a film, lowers the surface tension and makes two immiscible liquids miscible. A very important factor affecting the stability of the emulsion is the amount of the emulsifying agent. Emulsifiers have the property of reducing the oil/water or water/oil interfacial tension, improving the stability of the emulsion and also directly influencing the stability, sensory properties and surface tension of sunscreens by modulating the filmometric performance.
Medical
It is able to partially remove scars produced by acne.
Other uses
It is used in insecticides, water-soluble flavourings, fungicides, coolants, mosquito repellents.
Hydrogenated castor oil is a derivative of castor oil and is obtained by catalytic hydrogenation of castor oil. It significantly reduces the unsaturated double bond in castor oil molecules, so that it decreases the possibility of double bonds inducing yellowing of animal skins used for clothing (1).
An evaluation of the safe use of high concentrations of hydrogenated castor oil (PEG-40) as surfactant for oral nanoemulsion was performed in Webster mice. In particular, considering in vivo data, it is suggested that nanoemulsion composed with a high amount of hydrogenated castor oil PEG-40 is acceptable for the oral delivery of active compounds (2).
It improves the corrosion resistance of carbon steel in simulated fuel ethanol (3).
Used as an adjuvant for coatings.
Provides resistance to failure in thixotropic epoxy mortars and in solvent-based coatings, inks and adhesive systems.
For more information:
PEG-40 Hydrogenated Castor Oil studies
Typical commercial product characteristics Hydrogenated Castor Oil
| Appearance | Yellowish liquid or white flakes |
| pH | 5.0~7.0 |
| Boiling Point | 872.4±30.0°C at 760 mmHg (liquid) |
| Melting Point | ≥85°C (flakes) |
| Flash Point | 220.1±18.1°C (liquid) |
| Density | 1.0±0.1 g/cm3 |
| Refraction Index | 1.478 |
| Vapor Pressure | 0.0±0.6 mmHg at 25°C |
| LogP | 18.70 |
| Saponification value | 57~67 (liquid) 176-186 mgKOH/g (flakes) |
| HLB | 13~14 (liqiod) |
| Iodine | ≤ 2.5 gl/100g (flakes) 82-92 (liquid) |
| Acid Value | ≤ 2.0 Mg KOH/g (flakes) 175-185(liquid) |
| Particle size | dv98≤20 μm (flakes) |
| Hydroxy Value | ≥ 155 Mg KOH/g (flakes) |
| Water | 0.5% max (liquid) |
 |  |
 |  |
Synonyms :
References____________________________________________________
(1) Ma J, Duan L, Lu J, Lyu B, Gao D, Wu X. Fabrication of modified hydrogenated castor oil/GPTMS-ZnO composites and effect on UV resistance of leather. Sci Rep. 2017 Jun 16;7(1):3742. doi: 10.1038/s41598-017-03879-3.
(2) Rachmawati H, Novel MA, Ayu S, Berlian G, Tandrasasmita OM, Tjandrawinata RR, Anggadiredja K. The In Vitro-In Vivo Safety Confirmation of PEG-40 Hydrogenated Castor Oil as a Surfactant for Oral Nanoemulsion Formulation. Sci Pharm. 2017 Mar 31;85(2):18. doi: 10.3390/scipharm85020018.
(3) Deyab MA. Utilization of a nonionic surfactant for improved corrosion resistance of carbon steel in simulated fuel-grade ethanol. RSC Adv. 2018 Jun 7;8(37):20996-21001. doi: 10.1039/c8ra02936a.
_____________________________________________________________________________
And a premise on PEG.
Since the PEG (1) family is numerous and is found in many cosmetic, cleaning and medicinal products and others, we need a cognitive premise on the subject that is rather complex from the point of view of safety because these products not only come into contact with the skin but, as in the case of medicine, they are also ingested.
PEG or polyethylene glycols polymerise the condensed ethylene oxide and water and are called polyethylene glycols, but in reality, they are complex chemical components, polymers bound together. For example, plastic is polyethylene and has a hard consistency, while polyethylene aggregated to the glycol forms a liquid.
The number that appears after the initials PEG represents the molecular weight and the higher this number is, the less it penetrates the skin.
Here below are some studies in Medicine that refer to the use of PEG Polyethylene glycol in various fields.
Intestine
Polyethylene glycol with or without electrolytes is effective for the treatment of functional constipation, both in adults and in paediatric patients, with great safety and tolerability. These preparations are the most effective osmotic laxatives (more than lactulose) and are the first-line treatment for functional constipation in the short- and long-term. They are as effective as enemas in faecalomas, avoid the need for hospitalisation and are well tolerated by patients (especially when given without electrolytes) (2).
In the preparation for colonoscopy, polyethylene glycol tablets confirmed efficacy, acceptability, tolerance and safety similar to those of sodium phosphate (3).
For peripheral nerve repair (4).
Eyes
Dry eye syndrome is a disorder that affects 5-34% of the world's adult population with reduced quality of life. Artificial or lubricating tears are the most used therapy for treating this condition due to their low side effects profile, which attempt to modify the properties of the tear film. Polyethylene glycol has demonstrated clinical efficacy in the treatment of this condition (5).
Brain
Polyethylene glycol facilitates the neuroprotective effects of magnesium in head injuries (6).
Tumors
For transarterial chemoembolization, Polyethylene glycol is effective and safe for the treatment of liver cancer, as indicated by good tolerability, quality of life and high tumour response (7).
Cosmetics
Many types of PEG are hydrophilic and are used as creams, topical dermatological preparations and in cosmetic products such as surfactants, emulsifiers, detergents, humectants and skin conditioners.
Safety varies from type to type given the structural complexity (8).
References___________________________________________________________________
(1) Fruijtier-Pölloth C. Safety assessment on polyethylene glycols (PEGs) and their derivatives as used in cosmetic products. Toxicology. 2005 Oct 15;214(1-2):1-38. doi: 10.1016/j.tox.2005.06.001.
(2) Mínguez M, López Higueras A, Júdez J. Use of polyethylene glycol in functional constipation and fecal impaction. Rev Esp Enferm Dig. 2016 Dec;108(12):790-806. doi: 10.17235/reed.2016.4571/2016.
Santos-Jasso KA, Arredondo-García JL, Maza-Vallejos J, Lezama-Del Valle P. Effectiveness of senna vs polyethylene glycol as laxative therapy in children with constipation related to anorectal malformation. J Pediatr Surg. 2017 Jan;52(1):84-88. doi: 10.1016/j.jpedsurg.2016.10.021.
(3) Chaussade S, Schmöcker C, Toulemonde P, Muñoz-Navas M, O'Mahony V, Henri F. Phosphate tablets or polyethylene glycol for preparation to colonoscopy? A multicentre non-inferiority randomized controlled trial. Surg Endosc. 2017 May;31(5):2166-2173. doi: 10.1007/s00464-016-5214-1.
Tsunoda T, Sogo T, Iwasawa K, Umetsu S, Oikawa-Kawamoto M, Inui A, Fujisawa T. Feasibility and safety of bowel cleansing using low-volume polyethylene glycol with ascorbic acid before pediatric colonoscopy: A pilot study. Dig Endosc. 2017 Mar;29(2):160-167. doi: 10.1111/den.12756.
(4) Hoffman AN, Bamba R, Pollins AC, Thayer WP. Analysis of polyethylene glycol (PEG) fusion in cultured neuroblastoma cells via flow cytometry: Techniques & optimization. J Clin Neurosci. 2017 Feb;36:125-128. doi: 10.1016/j.jocn.2016.10.032.
(5) Pérez-Balbuena AL, Ochoa-Tabares JC, Belalcazar-Rey S, Urzúa-Salinas C, Saucedo-Rodríguez LR, Velasco-Ramos R, Suárez-Sánchez RG, Rodríguez-Carrizalez AD, Oregón-Miranda AA. Efficacy of a fixed combination of 0.09 % xanthan gum/0.1 % chondroitin sulfate preservative free vs polyethylene glycol/propylene glycol in subjects with dry eye disease: a multicenter randomized controlled trial. BMC Ophthalmol. 2016 Sep 20;16(1):164. doi: 10.1186/s12886-016-0343-9.
Labetoulle M, Messmer EM, Pisella PJ, Ogundele A, Baudouin C. Safety and efficacy of a hydroxypropyl guar/polyethylene glycol/propylene glycol-based lubricant eye-drop in patients with dry eye. Br J Ophthalmol. 2017 Apr;101(4):487-492. doi: 10.1136/bjophthalmol-2016-308608.
(6) Busingye DS, Turner RJ, Vink R. Combined Magnesium/Polyethylene Glycol Facilitates the Neuroprotective Effects of Magnesium in Traumatic Brain Injury at a Reduced Magnesium Dose. CNS Neurosci Ther. 2016 Oct;22(10):854-9. doi: 10.1111/cns.12591.
(7) Aliberti C, Carandina R, Sarti D, Mulazzani L, Catalano V, Felicioli A, Coschiera P, Fiorentini G. Hepatic Arterial Infusion of Polyethylene Glycol Drug-eluting Beads for Primary and Metastatic Liver Cancer Therapy. Anticancer Res. 2016 Jul;36(7):3515-21.
(8) Jang HJ, Shin CY, Kim KB. Safety Evaluation of Polyethylene Glycol (PEG) Compounds for Cosmetic Use. Toxicol Res. 2015 Jun;31(2):105-36. doi: 10.5487/TR.2015.31.2.105.
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.EvaluateClose | (0 comments) |
Read other Tiiips about this object in __Italiano (2)
Component type: Chemical Main substances: Last update: 2023-06-26 19:20:05 | Chemical Risk: |

