Check the ingredients!
... live healthy!


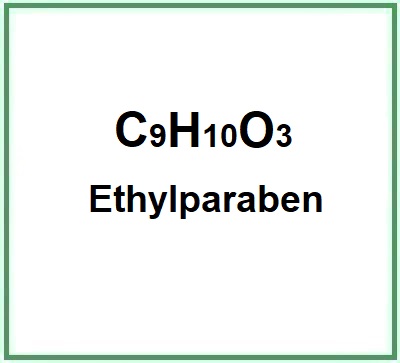
![]() Ethylparaben
Ethylparaben
Rating : 3.5
| Evaluation | N. Experts | Evaluation | N. Experts |
|---|---|---|---|
| 1 | 6 | ||
| 2 | 7 | ||
| 3 | 8 | ||
| 4 | 9 | ||
| 5 | 10 |
Cons:
Possible risk. Click ingredient (1)7 pts from FRanier
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.Evaluate | Where is this found? |
| "Ethylparaben studies" about Ethylparaben by Ark90 (12417 pt) | 2022-Sep-29 11:30 |
| Read the full Tiiip | (Send your comment) |
Compendium of the most significant studies with reference to properties, intake, effects.
Kim MJ, Kim CH, An MJ, Lee JH, Shin GS, Song M, Kim JW. Ethylparaben induces apoptotic cell death in human placenta BeWo cells via the Caspase-3 pathway. Anim Cells Syst (Seoul). 2020 Jan 9;24(1):34-43. doi: 10.1080/19768354.2020.1711804.
Abstract. Parabens are generally used as preservatives in foods, pharmaceuticals, and various other commercial products. Among them, ethylparaben has weaker estrogenic characteristics than endogenous estrogen. However, growing evidence indicates that ethylparaben has an adverse effect on various human tissues. Here, we investigated whether ethylparaben induces cell death by affecting cell viability, cell proliferation, cell cycle, and apoptosis using the human placenta cell line BeWo. Ethylparaben significantly decreased cell viability in a dose-dependent manner. It caused cell cycle arrest at sub-G1 by reducing the expression of cyclin D1, whereas it decreased the cell proportion at the G0/G1 and S phases. Furthermore, we verified that ethylparaben induces apoptotic cell death by enhancing the activity of Caspase-3. Taken together, our results suggest that ethylparaben exerts cytotoxic effects in human placental BeWo cells via cell cycle arrest and apoptotic pathways. © 2020 The Author(s). Published by Informa UK Limited, trading as Taylor & Francis Group Korean Society for Integrative Biology.
Golden R, Gandy J, Vollmer G. A review of the endocrine activity of parabens and implications for potential risks to human health. Crit Rev Toxicol. 2005 Jun;35(5):435-58. doi: 10.1080/10408440490920104.
Abstract. Parabens are a group of the alkyl esters of p-hydroxybenzoic acid and typically include methylparaben, ethylparaben, propylparaben, butylparaben, isobutylparaben, isopropylparaben, and benzylparaben. Parabens (or their salts) are widely used as preservatives in cosmetics, toiletries, and pharmaceuticals due to their relatively low toxicity profile and a long history of safe use. Testing of parabens has revealed to varying degrees that individual paraben compounds have weakly estrogenic activity in some in vitro screening tests, such as ligand binding to the estrogen receptor, regulation of CAT gene expression, and proliferation of MCF-7 cells. Reported in vivo effects include increased uterine weight (i.e., butyl-, isobutyl-, and benzylparaben) and male reproductive-tract effects (i.e., butyl- and propylparaben). However, in relation to estrogen as a control during in vivo studies, the parabens with activity are many orders of magnitude less active than estrogen. While exposure to sufficient doses of exogenous estrogen can increase the risk of certain adverse effects, the presumption that similar risks might also result from exposure to endocrine-active chemicals (EACs) with far weaker activity is still speculative. In assessing the likelihood that exposure to weakly active EACs might be etiologically associated with adverse effects due to an endocrine-mediated mode of action, it is paramount to consider both the doses and the potency of such compounds in comparison with estrogen. In this review, a comparative approach involving both dose and potency is used to assess whether in utero or adult exposure to parabens might be associated with adverse effects mediated via an estrogen-modulating mode of action. In utilizing this approach, the paraben doses required to produce estrogenic effects in vivo are compared with the doses of either 17beta-estradiol or diethylstilbestrol (DES) that are well established in their ability to affect endocrine activity. Where possible and appropriate, emphasis is placed on direct comparisons with human data with either 17beta-estradiol or DES, since this does not require extrapolation from animal data with the uncertainties inherent in such comparisons. Based on these comparisons using worst-case assumptions pertaining to total daily exposures to parabens and dose/potency comparisons with both human and animal no-observed-effect levels (NOELs) and lowest-observed-effect levels (LOELs) for estrogen or DES, it is biologically implausible that parabens could increase the risk of any estrogen-mediated endpoint, including effects on the male reproductive tract or breast cancer. Additional analysis based on the concept of a hygiene-based margin of safety (HBMOS), a comparative approach for assessing the estrogen activities of weakly active EACs, demonstrates that worst-case daily exposure to parabens would present substantially less risk relative to exposure to naturally occurring EACs in the diet such as the phytoestrogen daidzein.
Liu F, Kong X, Kong H. Ethylparaben induces subconjunctival fibrosis via the Wnt/β-catenin signaling pathway. Exp Ther Med. 2021 Apr;21(4):295. doi: 10.3892/etm.2021.9726.
Abstract. The aim of the present study was to explore the etiology of subconjunctival fibrosis (SCF) induced by ethylparaben, the most prevalent preservative in Chinese eye drops....Copyright © 2020, Spandidos Publications.
Wang D, Li W, Yang C, Chen X, Liu X, He J, Tong C, Peng C, Ding Y, Geng Y, Cao X, Li F, Gao R, Wang Y. Exposure to ethylparaben and propylparaben interfere with embryo implantation by compromising endometrial decidualization in early pregnant mice. J Appl Toxicol. 2021 Nov;41(11):1732-1746. doi: 10.1002/jat.4208.
Abstract. Ethylparaben (EtP) and propylparaben (PrP) are common preservatives and well-known endocrine-disrupting chemicals. Studies have demonstrated that they can reduce female fertility, but the underlying mechanism, especially that on embryo implantation, is still poorly understood. Endometrial decidualization is a critical event for embryo implantation. In this study, we aimed to explore the effects of EtP/PrP on endometrial decidualization.... © 2021 John Wiley & Sons, Ltd.
Sun L, Yu T, Guo J, Zhang Z, Hu Y, Xiao X, Sun Y, Xiao H, Li J, Zhu D, Sai L, Li J. The estrogenicity of methylparaben and ethylparaben at doses close to the acceptable daily intake in immature Sprague-Dawley rats. Sci Rep. 2016 Apr 28;6:25173. doi: 10.1038/srep25173.
Abstract. The estrogenicity of parabens at human exposure levels has become a focus of concern due to the debate over whether the estrogenicity of parabens is strong enough to play a role in the increased incidence of breast cancer. In this study, the uterotrophic activities of methylparaben (MP) and ethylparaben (EP) at doses close to the acceptable daily intake as allocated by JECFA were demonstrated in immature Sprague-Dawley rats by intragastric administration, and up-regulations of estrogen-responsive biomarker genes were found in uteri of the rats by quantitative real-time RT-PCR (Q-RT-PCR). At the same time, the urinary concentrations of MP and EP, as measured by gas chromatography-mass spectrometry (GC-MS) in rats that received the same doses of MP and EP, were found to be near the high urinary levels reported in human populations in recent years. These results show the in vivo estrogenicity of MP and EP at human exposure levels, and indicate that populations exposed to large amounts of MP and EP may have a high burden of estrogenicity-related diseases. In addition, a molecular docking simulation showed interaction between the parabens and the agonist-binding pocket of human estrogen receptor α (hERα).
Matwiejczuk N, Galicka A, Brzóska MM. Review of the safety of application of cosmetic products containing parabens. J Appl Toxicol. 2020 Jan;40(1):176-210. doi: 10.1002/jat.3917.
Abstract. Cosmetics are a source of lifetime exposure to various substances including parabens, being the most popular synthetic preservatives. Because the use of cosmetics shows an increasing trend and some adverse health outcomes of parabens present in these products have been reported, the present review focused on the safety of dermal application of these compounds. Special attention has been paid to the absorption of parabens and their retention in the human body in the intact form, as well as to their toxicological characteristics. Particular emphasis has been placed on the estrogenic potential of parabens....© 2020 John Wiley & Sons, Ltd.
Tavares RS, Martins FC, Oliveira PJ, Ramalho-Santos J, Peixoto FP. Parabens in male infertility-is there a mitochondrial connection? Reprod Toxicol. 2009 Jan;27(1):1-7. doi: 10.1016/j.reprotox.2008.10.002.
Abstract. Parabens are widely used as preservatives in many foods, cosmetics, toiletries, and pharmaceuticals due to their relatively low toxicity profile and to a long history of safe use. Parabens are alkyl esters of p-hydroxybenzoic acid and typically include methylparaben, ethylparaben, propylparaben, butylparaben, isobutylparaben, isopropylparaben and benzylparaben. These compounds are known to have a null or very weak estrogenic activity in estrogen receptor assays in vitro. In recent years, an increasing concern has emerged regarding possible adverse effects of chemicals in food and in cosmetics on human reproduction outcomes. In developed countries about 15% of human couples are affected by infertility, almost half of these cases attributed to men, through low sperm motility or/and sperm count. It is known that a significant number of cases of male infertility results from exposure to xenobiotics, and also that testis mitochondria are particularly affected by drug-induced toxicity. The present review discusses evidence that parabens may not be as safe as initially thought, and suggests that the interaction between parabens and mitochondrial function in the testis may be key in explaining the contribution of parabens for a decrease in reproductive potential.
Dogan S, Tongur T, Erkaymaz T, Erdogan G, Unal B, Sik B, Simsek T. Traces of intact paraben molecules in endometrial carcinoma. Environ Sci Pollut Res Int. 2019 Oct;26(30):31158-31165. doi: 10.1007/s11356-019-06228-1.
Abstract. Endometrial carcinoma is the most commonly encountered gynecological cancer in women worldwide and is also one of the popular models of the hormone-dependent carcinomas. This study was aimed to evaluate and compare the concentrations of five paraben molecules (methylparaben, ethylparaben, N-propylparaben, benzylparaben, isobutylparaben + N-butylparaben) in the endometrial and myometrial tissue samples of patients diagnosed with endometrial carcinoma and benign gynecologic diseases. A total of 88 patients were included in the study and chemical analysis was performed on 176 tissue samples. The study group comprised of 33 patients with endometrial carcinoma and 6 patients with endometrial intraepithelial neoplasia. The control group comprised of 49 patients. One endometrial and one myometrial tissue samples were collected from each patient. The analyses were performed using ultrahigh-performance liquid chromatography and tandem mass spectrometry (UHPLC-MS/MS). At least one type of paraben molecule was detected in 23.07% (9/39) of the patients in the study group, and in 2.04% (1/49) of the patients in the control group; this difference between the groups was statistically significant (p = .002). N-Propylparaben and isobutyl + N-butylparaben were the most frequently detected (in 7/10 of the samples) paraben molecules in the study. Tumor characteristics (tumor diameter, myometrial invasion, architectural grade, nuclear grade, lymphovascular space invasion, and tumor stage) were comparable between the two groups of endometrial carcinoma (paraben-detected and paraben-undetected groups). In conclusion, paraben molecules were more frequently detected in the endometrial carcinoma tissue samples than in the normal endometrium.
Finot F, Kaddour A, Morat L, Mouche I, Zaguia N, Cuceu C, Souverville D, Négrault S, Cariou O, Essahli A, Prigent N, Saul J, Paillard F, Heidingsfelder L, Lafouge P, Al Jawhari M, Hempel WM, El May M, Colicchio B, Dieterlen A, Jeandidier E, Sabatier L, Clements J, M'Kacher R. Genotoxic risk of ethyl-paraben could be related to telomere shortening. J Appl Toxicol. 2017 Jun;37(6):758-771. doi: 10.1002/jat.3425.
Abstract. The ability of parabens to promote the appearance of multiple cancer hallmarks in breast epithelium cells provides grounds for regulatory review of the implication of the presence of parabens in human breast tissue. It is well documented that telomere dysfunction plays a significant role in the initiation of genomic instability during carcinogenesis in human breast cancer. In the present study, we evaluated the genotoxic effect of ethyl 4-hydroxybenzoate (ethyl-paraben), with and without metabolic activation (S9), in studies following OECD guidelines....Copyright © 2016 John Wiley & Sons, Ltd.
Jensen TK, Andersson AM, Main KM, Johannsen TH, Andersen MS, Kyhl HB, Juul A, Frederiksen H. Prenatal paraben exposure and anogenital distance and reproductive hormones during mini-puberty: A study from the Odense Child Cohort. Sci Total Environ. 2021 May 15;769:145119. doi: 10.1016/j.scitotenv.2021.145119.
Abstract. Background: Parabens are added to personal care products as antimicrobial preservatives. They have been suggested to have endocrine disrupting abilities. Prenatal exposure to parabens has been associated with reproductive endpoints including reduced male anogenital distance (AGD, distance from anus to genitals), which is sensitive to prenatal anti-androgenic exposure. Objectives: To study the associations between maternal paraben concentrations in second trimester urine and AGD and reproductive hormone concentrations at 3 months of age in offspring....Copyright © 2021 The Authors.
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.EvaluateClose | (0 comments) |
| "Descrizione" about Ethylparaben Review Consensus 7 by FRanier (9976 pt) | 2022-Sep-29 11:34 |
| Read the full Tiiip | (Send your comment) |
Ethylparaben is a chemical compound, ethyl ester of p-hydroxybenzoic acid.
It takes the form of a fine white powder that is odourless and tasteless.

What it is used for and where
It is a preservative additive used in various fields including cosmetics and is used to combat bacteria, fungi, yeasts and moulds. It is labelled with the number E214 in the list of European additives as a preservative.
Safety
Parabens are a controversial component and their safety has been questioned, especially because of the damage they are said to cause to the aquatic environment where they are discharged after use (1). Parabens may also contribute to obesity (2).
Like all members of the paraben family, it can cause allergies.
Parabens
Parabens are chemical preservative compounds that have been the subject of attention in the scientific literature as possible endocrine disruptors (particularly propylparaben and butylparaben), i.e. with the possibility of damaging the hormone-producing glands in our bodies, particularly in the breasts. The 2004 study by Darbre et al. showed that parabens remain in our bodies as intact esters (3). Following this study, some of the scientific literature in 2005 and 2006 cast doubt on Darbre's conclusions and claimed they were limited. However, both the US FDA and the European SCCP authorised in 2006 the use of a single paraben in cosmetic products at a concentration of 0.4% and the use of total parabens at a concentration of 0.8% (4). However, there is no shortage of studies that consider the restrictions unnecessary: M. G. Kirchhof et al. in 2013 found that parabens are among the safest and most well-tolerated preservatives and that current data do not support drastic regulations or personal exposure restrictions (5). Darbre in 2014 published a further study in which he showed how parabens can cause DNA damage (6).
For more information: Ethylparaben studies
Typical optimal commercial product characteristics Ethylparaben
| Appearance | White fine powder |
| Boiling Point | 297.5±0.0°C at 760 mmHg |
| Melting Point | 114-117 °C |
| Flash Point | 120.3±12.6°C |
| Density | 1.2±0.1 g/cm3 |
| Heavy metal (in Pb)%: | 0.001 |
| Loss on drying% | 0.5 |
| Sulfate (as SO42-)% | 0.024 |
| Arsenic | 0.0001 |
| Ignition residue% | 0.05 |
| Vapour Pressure | 0.0±0.6 mmHg at 25°C |
| Index of Refraction | 1.539 |
| PSA | 46.53000 |
| LogP | 2.40 |
 |  |
 |  |
Synonyms:
References__________________________________________________________________
(1) Terasaki M, Abe R, Makino M, Tatarazako N. Chronic toxicity of parabens and their chlorinated by-products in Ceriodaphnia dubia. Environ Toxicol. 2015 May-Jun;30(6):664-73. doi: 10.1002/tox.21944.
Popa DS, Bolfa P, Kiss B, Vlase L, Păltinean R, Pop A, Cătoi C, Crişan G, Loghin F. Influence of Genista tinctoria L. or methylparaben on subchronic toxicity of bisphenol A in rats. Biomed Environ Sci. 2014 Feb;27(2):85-96. doi: 10.3967/bes2014.021.
(2) Hu P, Chen X, Whitener RJ, Boder ET, Jones JO, Porollo A, Chen J, Zhao L. Effects of parabens on adipocyte differentiation. Toxicol Sci. 2013 Jan;131(1):56-70. doi: 10.1093/toxsci/kfs262.
(3) Darbre PD, Aljarrah A, Miller WR, Coldham NG, Sauer MJ, Pope GS. Concentrations of parabens in human breast tumours. J Appl Toxicol. 2004 Jan-Feb;24(1):5-13. doi: 10.1002/jat.958.
(4) https://www.fda.gov/cosmetics/cosmetic-ingredients/parabens-cosmetics
Cosmetic Ingredient Review Expert Panel. Final amended report on the safety assessment of Methylparaben, Ethylparaben, Propylparaben, Isopropylparaben, Butylparaben, Isobutylparaben, and Benzylparaben as used in cosmetic products. Int J Toxicol. 2008;27(Suppl 4):1-82.
(5) Kirchhof MG, de Gannes GC. The health controversies of parabens. Skin Therapy Lett. 2013 Feb;18(2):5-7.
(6) Darbre PD, Harvey PW. Parabens can enable hallmarks and characteristics of cancer in human breast epithelial cells: a review of the literature with reference to new exposure data and regulatory status. J Appl Toxicol. 2014 Sep;34(9):925-38. doi: 10.1002/jat.3027.
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.EvaluateClose | (0 comments) |
Read other Tiiips about this object in __Italiano (2)
Component type: Chemical Main substances: Last update: 2022-09-29 11:03:09 | Chemical Risk: |