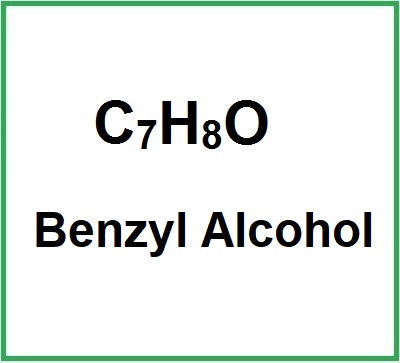
![]() Benzyl Alcohol
Benzyl Alcohol
Rating : 6.2
| Evaluation | N. Experts | Evaluation | N. Experts |
|---|---|---|---|
| 1 | 6 | ||
| 2 | 7 | ||
| 3 | 8 | ||
| 4 | 9 | ||
| 5 | 10 |
Pros:
Preservative (1)7 pts from A_Partyns
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.Evaluate | Where is this found? |
| "Benzyl Alcohol studies" about Benzyl Alcohol Review Consensus 10 by AColumn (9336 pt) | 2022-Nov-26 11:09 |
| Read the full Tiiip | (Send your comment) |
Compendium of the most significant studies with reference to properties, intake, effects.
Johnson W, Bergfeld WF, Belsito DV, Hill RA, Klaassen CD, Liebler DC, Marks JG, Shank RC, Slaga TJ, Snyder PW, Andersen FA. Safety Assessment of Benzyl Alcohol, Benzoic Acid and its Salts, and Benzyl Benzoate. Int J Toxicol. 2017 Nov/Dec;36(3_suppl):5S-30S. doi: 10.1177/1091581817728996.
Abstract. Benzyl alcohol, benzoic acid and its salts, and benzyl benzoate function mostly as fragrance ingredients/preservatives in cosmetic products. The Cosmetic Ingredient Review Expert Panel previously established concentration limits for benzyl alcohol, benzoic acid, and sodium benzoate in cosmetics and determined that the available data were insufficient to support the safety of these ingredients during inhalation exposure. After reviewing newly available data, it was concluded that benzyl alcohol, benzoic acid and its salts, and benzyl benzoate are safe in the present practices of use and concentration described in this safety assessment.
Wilson L, Martin S. Benzyl alcohol as an alternative local anesthetic. Ann Emerg Med. 1999 May;33(5):495-9. doi: 10.1016/s0196-0644(99)70335-5.
Abstract. Study objectives: Benzyl alcohol has been used as a local anesthetic for brief superficial skin procedures; however, its efficacy for long-term cutaneous anesthesia has not been established. We sought to compare the cutaneous anesthetic effects of benzyl alcohol with epinephrine with the effects of lidocaine with epinephrine and with placebo.....Conclusion: Benzyl alcohol with epinephrine provides prolonged cutaneous anesthesia, although it is not as effective as lidocaine with epinephrine. However, benzyl alcohol is significantly less painful on injection than lidocaine with epinephrine, and it may offer an alternative for local anesthesia.
Lam XM, Patapoff TW, Nguyen TH. The effect of benzyl alcohol on recombinant human interferon-gamma. Pharm Res. 1997 Jun;14(6):725-9. doi: 10.1023/a:1012190120061.
Abstract. Purpose: The goal of this study was to investigate the conformational change and aggregation of recombinant human interferon-gamma (rhIFN-gamma) as a result of interaction between benzyl alcohol and the protein. The effects of buffer concentration, buffer species, ionic strength, rhIFN-gamma and benzyl alcohol concentrations on the dynamics of the interaction in liquid formulations were also examined....Conclusions: Interaction between benzyl alcohol and rhIFN-gamma is formulation dependent. Protein concentration, buffer species, buffer concentration, and preservative concentration play a significant role in determining the extent of the interaction and consequently the stability of the product.
Kawai T, Yamauchi T, Miyama Y, Sakurai H, Ukai H, Takada S, Ohashi F, Ikeda M. Benzyl alcohol as a marker of occupational exposure to toluene. Ind Health. 2007 Jan;45(1):143-50. doi: 10.2486/indhealth.45.143.
Abstract. Benzyl alcohol (BeOH) is a urinary metabolite of toluene, which has been seldom evaluated for biological monitoring of exposure to this popular solvent. The present study was initiated to develop a practical method for determination of BeOH in urine and to examine if this metabolite can be applied as a marker of occupational exposure to toluene. A practical gas-liquid chromatographic method was successfully developed in the present study with sensitivity low enough for the application (the limit of detection; 5 microg BeOH /l urine with CV=2.7%). Linearity was confirmed up to 10 mg BeOH/l, the highest concentration tested, and the reproducibility was also satisfactory with a coefficient of variation of 2.7% (n=10). A tentative application of the method in a small scale study with 45 male workers [exposed to toluene up to 130 ppm as an 8-h time-weighted average (8-h TWA)] showed that BeOH in the end-of-shift urine samples was proportional to the intensity of exposure to toluene. The calculated regression equation was Y=50+1.7X (r=0.80, p<0.01), where X was toluene in air (in ppm as 8-h TWA) and Y was BeOH in urine (in microg/l of end-of-shift urine). The levels of BeOH in the urine of the non-exposed was about 50 microg/l, and ingestion of benzoate as a preservative in soft drinks did not affect the BeOH level in urine. The findings as a whole suggest that BeOH is a promising candidate for biological monitoring of occupational exposure to toluene.
Jaeschke H, Du K. Benzyl Alcohol: A novel treatment for acetaminophen overdose? Hepatology. 2015 Nov;62(5):1641-2. doi: 10.1002/hep.27786.
Abstract. We read with interest the recent paper by Cai et al. suggesting that “benzyl alcohol (BA) may prove to be a useful adjunct in the treatment of APAP and other forms of sterile liver injury”.1 We would like to point out a few reasons why BA will not likely be a clinically useful therapeutic option for APAP overdose patients. First, as the authors discussed themselves,1 BA can inhibit mitochondrial respiration and is toxic at higher doses. Second, 100 μM BA was shown to inhibit the enzyme activities of Cyp1A2, Cyp2E1 and Cyp3A4 by 20–50%.2 Since the authors used about 9 mM BA for the in vitro experiments and exposed the liver to mM concentrations in vivo,1 the protective effect was most likely caused by P450 inhibition. This conclusion is supported by the almost 100% protection in vivo and the fact that BA was most effective when given during a narrow window around the time of APAP treatment, which confirms that BA only protects when present during the metabolism phase of APAP. However, the established clinical antidote N-acetylcysteine is most effective during the metabolism phase and beyond with lower risk. Third, the authors argue about an inhibitory effect of BA on the inflammasome.1 Interestingly, all studies that investigated a role of the Nalp3 inflammasome and interleukin-1β (IL-1β) in APAP hepatotoxicity are in agreement that damage-associated-molecular-patterns released from necrotic cells activate macrophages to generate cytokines including pro-IL-1β.1,3,4 All groups also agree that inhibition or deficiency of caspase-1 eliminates processing of pro-IL-1β to the active cytokine.1,3,4 Most importantly, all agree that only a few pg/ml IL-1β are actually produced during APAP hepatotoxicity.1,3,4 However, the fact that mice treated with 10,000-fold more recombinant IL-1β had no effect on APAP-induced liver injury clearly indicates that minor quantities of endogenously generated IL-1β have no impact on APAP hepatotoxicity.4 Thus, there is little chance that the inflammasome and IL-1β will be clinically relevant targets. Case in point, in APAP overdose patients there is no significant IL-1β formation [Control: 3.8±0.5 pg/ml (n=3); APAP: 5.5±1.7 pg/ml (n=10)] and neutrophils do not contribute to the injury.10
Geier J, Ballmer-Weber B, Buhl T, Rieker-Schwienbacher J, Mahler V, Dickel H, Schubert S; IVDK. Is benzyl alcohol a significant contact sensitizer? J Eur Acad Dermatol Venereol. 2022 Jun;36(6):866-872. doi: 10.1111/jdv.17968.
Abstract. Background: Benzyl alcohol is a widely used preservative, solvent and fragrance material. According to published data, it is a rare sensitizer in humans. Objectives: To identify characteristics and sensitization patterns of patients with positive patch test reactions to benzyl alcohol and to check the reliability of the patch test preparation benzyl alcohol 1% pet....Conclusions: Sensitization to benzyl alcohol occurs very rarely, mainly in patients with stasis dermatitis. In view of our results, benzyl alcohol cannot be regarded as a significant contact allergen, and therefore marking it as skin sensitizer 1B and labelling it with H 317 is not helpful. © 2022 The Authors. Journal of the European Academy of Dermatology and Venereology published by John Wiley & Sons Ltd on behalf of European Academy of Dermatology and Venereology.
Meinking TL, Villar ME, Vicaria M, Eyerdam DH, Paquet D, Mertz-Rivera K, Rivera HF, Hiriart J, Reyna S. The clinical trials supporting benzyl alcohol lotion 5% (Ulesfia): a safe and effective topical treatment for head lice (pediculosis humanus capitis). Pediatr Dermatol. 2010 Jan-Feb;27(1):19-24. doi: 10.1111/j.1525-1470.2009.01059.x.
Abstract. Benzyl alcohol lotion 5% (BAL 5%) is a non-neurotoxic topical head lice treatment that is safe and effective in children as young as 6 months of age. The safety and efficacy of this pediculicide has been studied in 695 (confirm number) subjects in all phases of clinical development. Scanning electron micrographs (SEM) demonstrated that the active agent appears to stun the breathing spiracles open, enabling the vehicle to penetrate the respiratory mechanism (spiracles), therefore asphyxiating the lice. Initial phase II trials compared this novel product to RID using identical volumes of treatment (4 oz/application) and yielding, almost, identical efficacy. This outcome pointed to the significant importance of completely saturating the hair with the product in order to achieve maximum treatment success. A second phase II trial, which allowed the use of sufficient product to saturate the hair, resulted in 100% efficacy after both 10 and 30 minute treatments. A third phase II trial verified an effective dose. Phase III trials compared BAL 5% to vehicle placebo for two 10-minute applications. It proved to be safe and effective (p < 0.001) for treatment of head lice and is the first FDA-approved non-neurotoxic lice treatment, now available in the United States as Ulesfia lotion.
Cole LK, Luu DH, Rajala-Schultz PJ, Meadows C, Torres AH. In vitro activity of an ear rinse containing tromethamine, EDTA, and benzyl alcohol on bacterial pathogens from dogs with otitis. Am J Vet Res. 2006 Jun;67(6):1040-4. doi: 10.2460/ajvr.67.6.1040.
Abstract. Objective: To evaluate the in vitro activity of an ear rinse (ER) containing tromethamine, EDTA, and benzyl alcohol on bacterial pathogens from dogs with otitis....Conclusions and clinical relevance: On the basis of results of this study, future studies should be performed to evaluate the in vivo efficacy of ER alone as a treatment for otic infections caused by beta-hemolytic streptococcus, P aeruginosa, and Proteus spp and of ER combined with an antimicrobial agent for otic infections caused by Staphylococcus spp.
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.EvaluateClose | (0 comments) |
| "Benzyl alcohol safety" about Benzyl Alcohol Review Consensus 7 by AColumn (9336 pt) | 2021-Dec-21 10:31 |
| Read the full Tiiip | (Send your comment) |
Benzyl alcohol is a well-tolerated anaesthetic. Like other anaesthetics, it reversibly suppresses neuronal activity (1).
It protects against the hepatotoxicity of Acetaminophen which is a commonly used analgesic and antipyretic drug of which an overdose can cause severe liver injury and even liver failure resulting in 70,000 hospitalisations and 500 deaths each year in the USA, however Benzyl Alcohol itself is cytotoxic at high concentrations and is not effective in primary human hepatocytes (2).
In 2019, the European Union Panel on Food Additives and Flavourings (FAF) concluded the re-evaluation of Benzyl Alcohol in its use as a food additive stating that exposure to Benzyl Alcohol (E 1519) does not raise safety concerns for reported uses and use levels (3).
References__________________________________________________________________
(1) Al-Hasan YM, Krishnan HR, Ghezzi A, Prado FJ 3rd, Robles RB, Atkinson NS. Tolerance to anesthesia depends on synaptic proteins. Behav Genet. 2011 Sep;41(5):734-45. doi: 10.1007/s10519-011-9451-8.
(2) Du K, McGill MR, Xie Y, Jaeschke H. Benzyl alcohol protects against acetaminophen hepatotoxicity by inhibiting cytochrome P450 enzymes but causes mitochondrial dysfunction and cell death at higher doses. Food Chem Toxicol. 2015 Dec;86:253-61. doi: 10.1016/j.fct.2015.10.016.
(3) EFSA Panel on Food Additives and Flavourings (FAF), Younes M, Aquilina G, Castle L, Engel KH, Fowler P, Fürst P, Gürtler R, Gundert-Remy U, Husøy T, Mennes W, Moldeus P, Oskarsson A, Shah R, Waalkens-Berendsen I, Wölfle D, Boon P, Crebelli R, Di Domenico A, Filipič M, Mortensen A, Van Loveren H, Woutersen R, Gergelova P, Giarola A, Lodi F, Frutos Fernandez MJ. Re-evaluation of benzyl alcohol (E 1519) as food additive. EFSA J. 2019 Oct 30;17(10):e05876. doi: 10.2903/j.efsa.2019.5876.
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.EvaluateClose | (0 comments) |
| "Descrizione" about Benzyl Alcohol Review Consensus 7 by A_Partyns (12948 pt) | 2023-Nov-21 12:06 |
| Read the full Tiiip | (Send your comment) |
Benzyl alcohol is a natural aromatic alcohol. It occurs as a clear, colourless, oily liquid. It is soluble in water at a ratio of 1:30 and miscible with alcohol, ether and chloroform.
Raw Materials Used in the Production
Raw Materials: Benzyl alcohol can be produced from benzaldehyde, which is obtained from the oxidation of toluene, and hydrogen.
Functions of Raw Materials:
- Benzaldehyde: Serves as a chemical precursor for the production of benzyl alcohol.
- Hydrogen: Used in the reduction reaction of benzaldehyde to form benzyl alcohol.
Industrial Chemical Synthesis Process
- Preparation: Benzaldehyde is prepared through the oxidation of toluene.
- Reaction: Benzaldehyde is then reduced with hydrogen to form benzyl alcohol.
- Purification: The produced benzyl alcohol is purified to remove impurities.
It appears as a clear oily liquid.

What it is used for and where it is used
Medical
Benzyl alcohol is the most frequently used antimicrobial preservative in multidose protein formulations, typically at a concentration of 0.9% (1). Anaesthetic (2), bacteriostatic agent. Solvent for vitamin B injections
Cosmetics
Flavouring agent, solvent, preservative, viscosity decreasing agent, perfume fixative. Deodorant.
External analgesic. It is used for the temporary relief of itching and pain associated with mild skin irritation. The purpose of the external analgesic is not absorption through the skin, but the creation of a skin sensation that has the ability to relieve pain with a warm or cool feeling.
Fragrance. It plays a very important role in the formulation of cosmetic products as it allows perfume to be enhanced, masked or added to the final product, improving its commercial viability. The consumer always expects to find a pleasant scent in a cosmetic product.
Oral care agent. This ingredient can be placed in the oral cavity to improve and/or maintain oral hygiene and health, to prevent or improve a disorder of the teeth, gums, mucous membrane.
Preservative. Any product containing organic, inorganic compounds, water, needs to be preserved from microbial contamination. Preservatives act against the development of harmful microorganisms and against oxidation of the product.
Solvent. It is the substance for dissolving or dispersing surfactants, oils, dyes, flavourings, bactericidal preservatives in solution.
Viscosity decreasing Agent. Since viscosity is important for increasing the chemical and physical stability of the product, Viscosity Reducing Agent is an important dosage factor in gels, suspensions, emulsions, solutions.
Pharmaceuticals
Bacteriostatic agent in parenteral preparations, in water used to flush intravascular catheters, in the reconstitution of drugs. Excipient, solvent with medium boiling point.
Food
Flavouring agent as it is extracted from ylan-ylang, violet, jasmine and other flowers. On the European food additives list it has the number E1519.
In 2019, the European Union's Scientific Panel on Food Additives and Flavourings (FAF) concluded the re-evaluation of benzyl alcohol in its use as a food additive by stating that exposure to benzyl alcohol (E 1519) does not raise safety concerns for reported uses and use levels (3) and set an ADI (Acceptable Daily Intake) of 4 mg/kg body weight per day.
Other applications
- Solvent for epoxy resin, casein, synthetic paints and glass, dye for optical lenses.
- Widely used in the production of oil for ballpoint pens.
- Chromatographic analysis
Warnings
Benzyl alcohol generates a small amount of benzaldehyde and benzyl ether and is therefore not suitable for long-term storage as it can slowly oxidise.
The most relevant studies on the subject have been selected with a summary of their contents:
Optimal typical characteristics of the commercial product Benzyl alcohol
| Appearance | Clear, colourless, oily liquid |
| Boiling Point | 204.7±0.0 °C at 760 mmHg |
| Purity | 99% |
| Melting Point | -15 °C |
| Flash Point | 93.9±0.0 °C |
| Benzaldehyde (ppm) | 250max |
| Acidity(As benzoic acid)% | 0.1max |
| Cloride % | 0.005max |
| Specific Gravity (20 ℃) | 1.043-1.048 |
| Refractive index (20 ℃) | 1.538-1.541 |
| Water% | 0.1max |
| Water Solubility | 4.29 g/100 mL (20 ºC) |
| Residue on evaporation,% | 0.1max |
| PSA | 20.23000 |
| LogP | 1.03 |
| Vapour density | 3.7 |
| Self-ignition point | 435℃ |
| Storage | +2°C to +25°C |
| Safety |  |
 |  |
 |  |
- Molecular Formula: C7H8O C6H5CH2OH
- Molecular Weight: 108.14 g/mol
- Exact mass 108.057518
- CAS: 100-51-6
- EC Number:202-859-9
- UNIII:LKG8494WBH
- InChI=1S/C7H8O/c8-6-7-4-2-1-3-5-7/h1-5,8H,6H2
- InChl Key WVDDGKGOMKODPV-UHFFFAOYSA-N
- SMILES C1=CC=C(C=C1)CO
- IUPAC phenylmethanol
- ChEBI 17987
- ICSC 0833
- NSC 760098 8044
- RTECS DN3150000
- JECFA 25
- NCI
- DSSTox Substance ID DTXSID5020152
- Beilstein Registry Number:878307
- MDL number:MFCD00004599
- PubChem Substance ID:57648443
- FEMA Number: 2137
Synonyms:
- benzenemethanol
- phenylmethanol
- Benzoyl alcohol
- Hydroxytoluene
- Phenolcarbinol
- Benzenecarbinol
- Phenyl-Methanol
- Phenylmethyl alcohol
- alpha-Toluenol
- SCHEMBL147
References____________________________________________________________________
(1) Meyer BK, Ni A, Hu B, Shi L. Antimicrobial preservative use in parenteral products: past and present. J Pharm Sci. 2007 Dec;96(12):3155-67. doi: 10.1002/jps.20976.
(2) Spence S, Houslay MD. The local anaesthetic benzyl alcohol attenuates the alpha 2-adrenoceptor-mediated inhibition of human platelet adenylate cyclase activity when stimulated by prostaglandin E1, but not that stimulated by forskolin. Biochem J. 1989 Dec 1;264(2):483-8. doi: 10.1042/bj2640483.
(3) EFSA Panel on Food Additives and Flavourings (FAF), Younes M, Aquilina G, Castle L, Engel KH, Fowler P, Fürst P, Gürtler R, Gundert-Remy U, Husøy T, Mennes W, Moldeus P, Oskarsson A, Shah R, Waalkens-Berendsen I, Wölfle D, Boon P, Crebelli R, Di Domenico A, Filipič M, Mortensen A, Van Loveren H, Woutersen R, Gergelova P, Giarola A, Lodi F, Frutos Fernandez MJ. Re-evaluation of benzyl alcohol (E 1519) as food additive. EFSA J. 2019 Oct 30;17(10):e05876. doi: 10.2903/j.efsa.2019.5876.
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.EvaluateClose | (0 comments) |
Read other Tiiips about this object in __Italiano (3)
Component type: Chemical Main substances: Last update: 2022-11-26 10:31:22 | Chemical Risk: |