Hydroxyapatite is a mineral composed mainly of calcium and the essential component of tooth enamel and it is also the shield which acts as a shield to caries.
What is it for?
- Bone Repair and Regeneration: Hydroxyapatite is used in orthopedic and dental surgery for bone repair and replacement. It's particularly useful in treating critical-sized bone defects where natural healing is not possible. It's also used in the creation of scaffolds that mimic the architecture of human bone, providing a structure that new bone cells can grow on.
- Calcium Supplementation: Hydroxyapatite can be used as a source of calcium in dietary supplements. This is particularly useful for maintaining bone health and preventing conditions like osteoporosis.
- Drug Delivery: Hydroxyapatite can act as a carrier for drug delivery, especially for delivering drugs to bones. The structure of hydroxyapatite allows it to carry a variety of substances, and it's been explored for targeted drug delivery.
- Dental Applications: Hydroxyapatite is used in toothpaste and other oral hygiene products to help prevent cavities. It can also be used in dental surgery for tooth repair and replacement.
- Tissue Engineering: Hydroxyapatite is used in the development of scaffolds for tissue engineering. These scaffolds provide a structure for cells to grow on, helping to form new tissue.
- Protein or Drug Carriers: Hydroxyapatite has been used as a carrier for proteins or drugs due to its bioactivity and biocompatibility. It can adsorb and release active molecules, which is beneficial for various biomedical applications.
It is classified as bioactive, ie it has the capacity to help bone growth in applications
- orthopedic
- dental
- maxillofacial
It appears in the form of a white powder.

Food additive : "Hydroxyapatite (Food additive)"
Significant studies
Although animal studies revealed a high risk of bias and results should be interpreted with caution, the literature suggests that non-critical bone defects may heal spontaneously and without the need of a bone graft. Conversely, when critical-size defects are present, the use of hydroxyapatite bone graft improves the bone repair process (1).
Bone grafts are widely used for augmentation procedures in oral and maxillofacial surgery, with autogenous bone being the gold standard. Recently, the focus of research has shifted towards synthetic bone substitutes, as no second surgery is needed and large quantities of graft can easily be provided. Within the broad range of bone substitutes, synthetic hydroxyapatite has drawn much attention, as they are considered to be biocompatible, non-immunogenic, osteoconductive and osteoinductive. Scope of this review is to summarize existing knowledge concerning the molecular, cellular and pharmaceutical aspects of synthetic bone substitutes for oral and maxillofacial grafting (2).
Reconstructive surgery is presently struggling with the problem of infections located within implantation biomaterials. Of course, the best antibacterial protection is antibiotic therapy. However, oral antibiotic therapy is sometimes ineffective, while administering an antibiotic at the location of infection is often associated with an unfavourable ratio of dosage efficiency and toxic effect. Thus, the present study aims to find a new factor which may improve antibacterial activity while also presenting low toxicity to the human cells. Such factors are usually implemented along with the implant itself and may be an integral part of it. Many recent studies have focused on inorganic factors, such as metal nanoparticles, salts, and metal oxides. The advantages of inorganic factors include the ease with which they can be combined with ceramic and polymeric biomaterials. The following review focuses on hydroxyapatites substituted with ions with antibacterial properties. It considers materials that have already been applied in regenerative medicine (e.g., hydroxyapatites with silver ions) and those that are only at the preliminary stage of research and which could potentially be used in implantology or dentistry. We present methods for the synthesis of modified apatites and the antibacterial mechanisms of various ions as well as their antibacterial efficiency (3).
See also : "Zinc Hydroxyapatite"
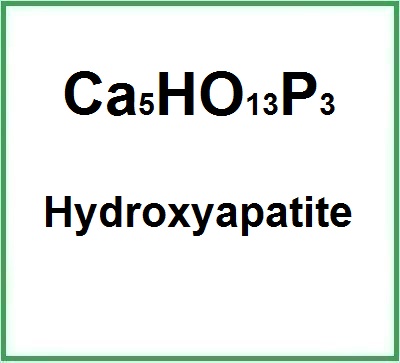
Molecular Formula (Ca5(OH)(PO4)3) Ca5HO13P3 Ca5(PO4)3(OH)
Molecular Weight 502.306 g/mol
CAS 1306-06-5 12167-74-7
Synonyms:
- Calcium hydroxyapatite
- Calcium phosphate hydroxide
- calcium hydroxide tris(phosphate)
- pentacalcium(2+) OH- triphosphate
- Pentacalcium hydroxide tris(orthophosphate)
- Decacalcium hexaphosphate dihydroxide
- Durapatite
- Hydroxylapatite
- EINECS 215-145-7
- EINECS 235-330-6
References_________________________________________________________________________
(1) Oliveira HL, Da Rosa WLO, Cuevas-Suárez CE, Carreño NLV, da Silva AF, Guim TN, Dellagostin OA, Piva E. Histological Evaluation of Bone Repair with Hydroxyapatite: A Systematic Review. Calcif Tissue Int. 2017 Oct;101(4):341-354. doi: 10.1007/s00223-017-0294-z. Epub 2017 Jun 13. Review.
(2) Gotz W, Papageorgiou SN. Molecular, Cellular and Pharmaceutical Aspects of Synthetic Hydroxyapatite Bone Substitutes for Oral and Maxillofacial Grafting. Curr Pharm Biotechnol. 2017;18(1):95-106. doi: 10.2174/1389201017666161202103218. Review.
(3) Kolmas J, Groszyk E, Kwiatkowska-Różycka D. Substituted hydroxyapatites with antibacterial properties. Biomed Res Int. 2014;2014:178123. doi: 10.1155/2014/178123. Epub 2014 May 11. Review.
![]() Durapatite
Durapatite